Introduction
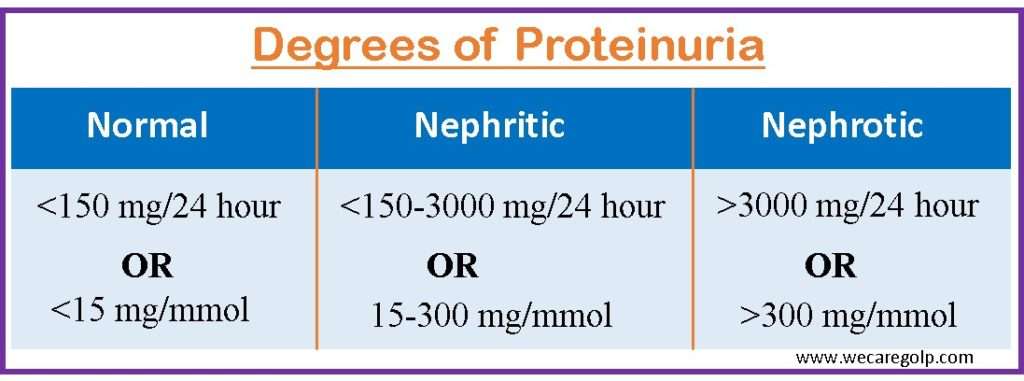
Proteinuria is a general term that refers to protein in the urine. It refers to the presence of proteins in the urine, such as albumin, globulin, Bence-Jones protein, and mucoprotein. The distal tubule produces Tamm-Horsfall glycoprotein, which accounts for over half of the protein lost in normal urine. Any number higher than the average daily excretion of total urine protein, which is around 150 mg/day, is regarded as proteinuria.
- It is a common occurrence in both outpatient and inpatient settings. Any such findings warrant additional examination, especially in the context of comorbidity. The incidence of proteinuria has become directly proportional to the incidence of diabetes.
- It is now used in the categorization of chronic kidney disease (CKD) with estimated glomerular filtration rate (eGFR).
- It is a marker for kidney disease progression, and higher cardiovascular morbidity in individuals. A person who has proteinuria and a normal glomerular filtration rate (GFR) is at a very high risk of gradually losing their kidney function.
- It might be a sign of early renal dysfunction. It denotes a greater risk of kidney injury as a result of hypertension and cardiovascular disease. It severity corresponds with disease progression.
- An albumin excretion rate (AER) of greater than 30 mg/day is regarded as abnormal, with the usual range being 5 to 10 mg/day. Moderately elevated albuminuria is defined as AER between 30 and 300 mg/day. Severely elevated albuminuria is defined as levels higher than 300 mg/day.
- CKD is characterized by prolonged albuminuria of three months or more. More than 3.5 g of protein produced in the urine during 24-hours is referred to as nephrotic-range proteinuria.
Incidence
- In the general community, proteinuria affects between 8% and 33% of people.
- The majority of primary and secondary renal disorders affect men more frequently than women.
- Up to 40% of patients with type 1 diabetes and up to 30% of persons with type 2 diabetes experience proteinuria at some time throughout their condition.
- Males get persistent proteinuria twice as frequently as females.
- Persistent proteinuria and microalbuminuria rates rise with age due to the rise in the prevalence of hypertension and diabetes.
Classification of Proteinuria
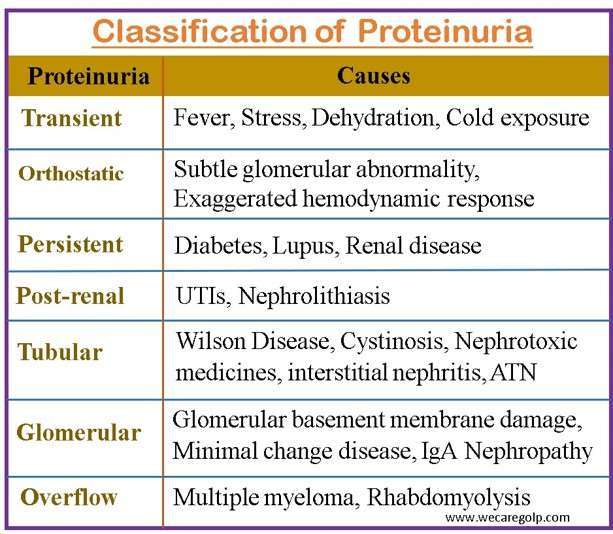
Transient proteinuria
- Temporary protein excretion, or transient proteinuria, can result after strenuous exercise, a high temperature, exposure to the cold, stress, and other situations.
- The amount of protein excreted in urine by pregnant women may also increase.
- Treatment is not necessary for transient proteinuria since there is no underlying renal disease.
Orthostatic proteinuria:
- It is often referred to as postural proteinuria, which is a disorder where the patient excretes a normal quantity of protein when they are supine but an unusually high amount of protein when they are upright.
- It occurs rarely after the age of 30 and affects 2 to 5% of adolescents. It is a minor condition.
- Thus, a correct diagnosis of orthostatic proteinuria is crucial in clinical practice to avoid doing any needless procedures like kidney biopsies.
Persistent proteinuria
- A person with persistent proteinuria has high quantities of protein in their urine for a prolonged period of time, usually more than three months. It frequently indicates an underlying medical emergency, such as diabetes, lupus, or renal disease.
- Damage to the glomeruli, which are small blood arteries in the kidneys that filter waste and extra fluid from the blood, can result in it.
- Damage to these vessels can cause the protein to flow into the urine, which can cause long-term proteinuria. High blood pressure, infections, and certain drugs are other causes that might cause it.
Post-renal proteinuria
- It is associated with urinary tract inflammation.
- Urinary tract infection, nephrolithiasis, and urinary tract malignancies are common disorders hypothesized to be related to post-renal proteinuria.
- As the underlying problem cures, it typically disappears as well.
Tubular proteinuria
- It is caused by tubulointerstitial disease, which affects the proximal renal tubules and interstitium.
- This reduces the proximal reabsorption of proteins, especially proteins with a low molecular weight (typically less than 25,000 Daltons), such as beta-2 microglobulin.
- It can occur in both inherited and acquired tubular diseases. RTA, cystinosis, Wilson disease, Fanconi syndrome, Lowe syndrome, Dent disease, and nephronophthisis are all hereditary causes of tubular proteinuria.
- Nephrotoxic medicines, interstitial nephritis, reflux nephropathy, acute tubular necrosis (ATN), and heavy metal toxicity are examples of acquired diseases.
- It is distinguished by the presence of 25%-60% low molecular weight proteins.
- When the tubulointerstitial compartment is injured, both the proteins that are typically filtered and reabsorbed as well as those produced by tubular epithelial cells in response to the damage are lost.
- Proteinuria is assumed to be caused by a failure of tubular reabsorption of plasma proteins. These proteins are entirely reabsorbed in the proximal tubules under normal circumstances. Proteinuria is typically 2 g/day, and dipstick findings may be negative.
Glomerular proteinuria
- Glomerular proteinuria is caused by an increase in macromolecule filtration (such as albumin) over the glomerular capillary wall. This is a highly sensitive indicator of the existence of glomerular disease.
- It is divided into two types: selective (albumin and transferrin in urine) and nonselective (all proteins are present). Albumin is always the most abundant protein in glomerular proteinuria.
- Total protein concentration is normally within 1500 mg/24 h urine in mild glomerular proteinuria, whereas in severe glomerular proteinuria, total protein concentration can range between 1500 and 3000 mg/24 h. Total protein in urine typically reaches 3000 mg/24 h in nonselective proteinuria.
Overflow proteinuria
- It is most typically related to the excessive synthesis of inappropriate low molecular weight proteins (e.g., light chains in multiple myeloma, myoglobin in rhabdomyolysis) that surpasses the proximal tubule’s reabsorption capacity, resulting in protein spilling into the urine.
- These low-molecular-weight proteins are toxic to the tubules and can result in acute kidney damage. Paraprotein deposition, for example, can cause glomerulopathy, resulting in increased albumin loss and more severe proteinuria.
Causes of Proteinuria
Transient proteinuria
- Fever
- Heavy exercise
- Congestive heart failure
- Epinephrine therapy
- Dehydration
- Cold exposure
- Emotional stress
- Seizures
- Pregnancy
- Vaginal mucus
- Urinary tract infection
Primary renal disease
- Glomerulonephritis
- Immunoglobulin A (IgA) nephropathy
- Focal segmental glomerulosclerosis
- Minimal change disease
- Membranoproliferative glomerulonephritis (MPGN)
- Mesangial proliferative glomerulonephritis
- Fibrillary glomerulonephritis
- Immunotactoid glomerulonephritis
- Kidney cancer
Secondary renal disease
- Diabetes mellitus
- Connective tissue diseases
- Vasculitis
- Bacterial endocarditis
- Henoch-Schönlein purpura
- Postinfectious glomerulonephritis
- Amyloidosis
- Myeloma
- Congestive cardiac failure
- Hypertension
Tubulointerstitial disease
- Tubulointerstitial nephritis
- Cystinosis
- Lowe syndrome
- Reflux nephropathy
- Renal hypoplasia/dysplasia
- Ischemic tubular injury
Other causes
- Medications: Heroin, interferon alfa, lithium, nonsteroidal anti-inflammatory drugs, pamidronate, sirolimus
- Infections: Herpes zoster, hepatitis B, Hepatitis C, Epstein-Barr virus
- Neoplasms: Leukemia, Melanoma, Lymphomas
- Autoimmune disorders: Systemic lupus erythematosus (SLE), Goodpasture Syndrome
- Hereditary nephritis, Alport syndrome
- Castleman disease
- Malignant hypertension
- Pre-eclampsia
- Transplant glomerulopathy
Signs and Symptoms of Proteinuria
Most people with proteinuria are asymptomatic, and the condition is first discovered by urine testing as part of the assessment for a significant life event or a high-risk person screening process.
- Foamy or frothy urine
- Edema (Face, stomach, feet, or ankles)
- Polyuria
- Shortness of breath
- Fatigue
- Nausea or vomiting
- Muscle cramping
- Dysuria
- Hyperlipidemia
- Backache
- Reduced GFR
- Hematuria
Pathophysiology of Proteinuria
- The pathophysiology can be described with the following two mechanisms.
- Abnormal transglomerular protein passage caused by increased permeability of the glomerular capillary wall
- Poor reabsorption by proximal tubular epithelial cells
- The severity of disruption of the structural integrity of the glomerular capillary wall corresponds with the area of the glomerular barrier being pierced by “big” holes, allowing the passage in the tubular lumen of high-molecular-weight (HMW) proteins, to which the barrier is ordinarily impermeable.
- Under normal physiological conditions, proteins cannot normally enter the urine because of the kidneys’ glomerular filtration barrier (GFB).
- The glomerular basement membrane, fenestrated endothelial cells, and podocytes (epithelial cells) make up the GFB. The glomerular filtration barrier’s negative charge and size selectivity prevent proteinuria.
- Crosstalk between the endothelium, mesangium, and podocytes keeps the filtration barrier functioning normally.
- Due to their interdependence, any damage to one of them will have an impact on how well the others work.
- The increased load of these proteins in the tubular lumen results in the tubular cells’ toxic damage and saturation of the reabsorptive mechanism, which favors the increased excretion of all proteins in the urine, including low-molecular-weight (LMW) proteins, which are completely reabsorbed under physiological conditions.
Glomerular proteinuria
- Glomerular proteinuria is caused by a faulty glomerular filtration barrier or an increase in hydrostatic pressure. The failure of the charge barrier, which is made up of collagen and laminin, causes a loss of negative charge, which emerges as the presence of negatively charged proteins in the urine.
- Proteinuria can also be caused by mesangial cell proliferation in the glomerulus, extracellular matrix synthesis, and inflammatory cell infiltration. Proteinuria is prevented by podocytes, and molecular malfunction of nephrin and podocin at the podocyte level can result in proteinuria.
- TRPC (transient receptor potential cation) is a protein involved in calcium influx that has been linked to podocyte damage via an NFAT-mediated signaling pathway.
Tubulointerstitial dysfunction
- Tubulointerstitial Dysfunction is caused by proximal tubule malfunction, which impairs the intake of filtered proteins.
- It has less than 2 grams of protein in urine in 24 hours and is denoted as less severe proteinuria than glomerular failure.
- Smaller, positively charged proteins that are normally filtered via the glomerular capillary are lacking in urine due to practically full reabsorption by tubular epithelial cells.
- The proximal convoluted tubules are responsible for most of the protein reabsorption.
- This reabsorption has a transport maximum in the proximal convoluted tubules that, when reached, might result in proteinuria.
Diagnosis of Proteinuria
History
- Edema (leg, face swelling)
- Weight changes
- Symptoms of connective tissue diseases (arthralgias, skin rashes, and mouth ulcers)
- Pain (loin pain, abdominal pain, pleuritic chest pain)
- Shortness of breath
- The appearance of urine (red, smoky, frothy)
- Comorbidities (hypertension, diabetes mellitus, heart failure)
- Nephrotoxic drugs
- Non-steroidal anti-inflammatory drugs (NSAIDs)
- Antihypertensive drugs (including angiotensin-converting enzyme (ACE) inhibitors, and loop diuretics)
- Antibiotics (penicillamine, aminoglycosides)
- Family history of renal and connective tissue disorders
Physical examination
- Edema
- Muscle wasting
- Rashes
- Abdominal bruits
- Splinter hemorrhages
- Signs of systemic disease
- Retinopathy
- Joint swelling or deformity
- Stigmata of chronic liver disease
- Cardiac murmurs
- Organomegaly
- Lymphadenopathy
- High blood pressure
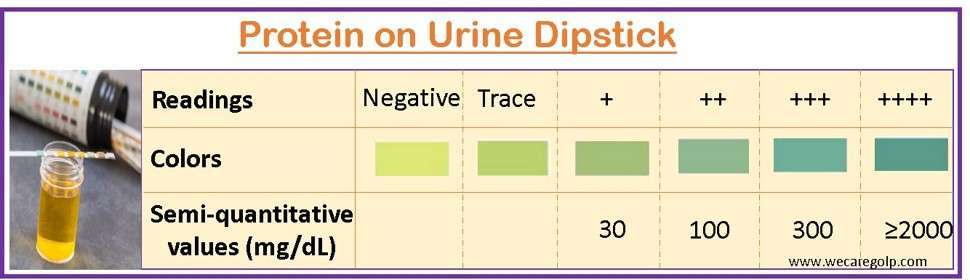
Urine dipstick
- The urine dipstick is simple and the first screening test for proteinuria that can be performed in an outpatient department.
- A specially treated paper strip is dipped into a urine sample.
- The color change (blue-green) on the strip indicates the presence and approximate amount of protein in the urine.
- However, it does not accurately quantify the amount of protein present or identify the specific proteins.
24-hour urine collection
A 24-hour urine collection is completely accurate for determining the degree of proteinuria; however, it is difficult to determine the 24-hour urinary protein excretion in mg per 24-hour period.
- Any reading above 150 mg/24 hours is regarded as abnormal and must be further examined for underlying causes.
- Errors in over- or under-collection might occur throughout the 24-hour urine collection.
Urine protein to creatinine ratio (UPCR)
- The spot urine protein to creatinine ratio (UPCR) or albumin to creatinine ratio (ACR) from a single specimen, ideally the early morning urine sample, is a quicker and more dependable alternative.
- Further investigation is needed if the UPCR value is higher than 15 mg/mmol.
- It can be calculated: (mg/l protein)/(mmol/l creatinine)*10.

Creatinine clearance
- The results are considered credible when 24-hour urinary protein excretion is compared to the average quantity of creatinine clearance per kilogram of lean body mass.
- Males secrete 20-25 mg/kg every day on average, whereas females secrete 15-20 mg/kg. Nevertheless, because lean body muscle mass declines after the age of 50, these estimations may be inaccurate in elderly individuals.
Microscopic Urinalysis
- Under a microscope, abnormal structures like red blood cells (RBCs), white blood cells (WBCs), or casts can be seen in a urine sample.
- Combining these findings with proteinuria can provide insights into the possible underlying causes.
Blood tests
- Serum electrolytes
- Urea
- Creatinine levels
- The blood albumin levels and cholesterol concentrations should be examined for nephrotic range proteinuria with more than 3.5 g/24-hours or a UPCR of more than 350 mg/mmol.
- Proteinuria should be evaluated in relation to renal function tests.
Imaging
- Ultrasounds, MRI, and CT scans can evaluate the kidney and detect any structural abnormalities (tumors, kidney stones, and other blockages) or kidney diseases that could be causing proteinuria.
- A small amount of kidney tissue is taken through renal biopsy and examined under a microscope to pinpoint the root of proteinuria.
- It should be considered in individuals with proteinuria of more than 1 g per day.
- It is also indicated when the underlying cause is not apparent since it can help guide therapy selection.
Others
- Autoantibody determinations
- Antistreptolysin O titers
- Antinuclear antibodies (ANAs)
- Anti-DNA antibodies
- Complement levels (C3 and C4)
- Anti-phospholipase A1 receptor autoantibody
- Cryoglobulins
- Urine and plasma protein electrophoresis for light chains
- Anti–glomerular basement membrane (anti-GBM) antibodies
- Antineutrophil cytoplasmic antibodies (ANCA)
- Hepatitis B and C and HIV serologies
Treatment of Proteinuria
The aims of the treatment of proteinuria
- To determine the underlying cause and, where feasible, treat it
- To reduce the likelihood of renal function decline by lowering proteinuria and managing hypertension
- To reduce the chance of developing cardiovascular disease
- To get patients who have advanced illnesses ready for renal replacement treatment (hemodialysis, peritoneal dialysis, or kidney transplant)
Pharmacological Management
- The specific underlying cause is the major focus of treatment.
- Also, most treatment approaches concentrate on lowering the level of proteinuria, especially albuminuria.
Angiotensin receptor antagonists (ARBs) and angiotensin-converting enzyme (ACE) inhibitors
- ARBs and ACE inhibitors are the first-line therapy for proteinuria.
- These medicines affect the renin-angiotensin-aldosterone system (RAAS).
- Adults with persistent proteinuria of more than 300 mg/24 hours are highly advised to use ACE inhibitors or ARBs (Kidney Disease Improving Global Outcomes (KDIGO) guideline).
- Patients who have moderate to severe proteinuria are often fluid-overloaded, necessitating diuretic medication as well as dietary salt reduction.
- Some people may not respond to standard diuretic dosages while having healthy kidneys and may need higher doses to reach the renal tubule.
- A combination of diuretics operating at several areas of the nephron might be used if fluid excess proves resistant to treatment with a single diuretic drug.
- Aggressive diuresis may increase the patient’s risk of acute kidney damage if the edema is brought on by marked hypoalbuminemia owing to intravascular volume depletion.
Aldosterone antagonist
- Aldosterone antagonist (spironolactone) has furthermore proven to be effective for treating proteinuria.
- ACE inhibitor and aldosterone antagonist combination treatment is linked to an increased risk of hyperkalemia and gynecomastia.
- Yet, individuals with heart failure who received this combination showed considerable mortality improvements.
Anticoagulants
- Patients are at risk of thrombosis and emboli if there is protein loss in the urine, particularly anticoagulant proteins such as antithrombin III, protein S, and protein C.
- Patients with severe albuminuria (serum albumin 2.5 g/dL) are advised to take warfarin.
Calcium channel blockers
- Diltiazem and verapamil, two calcium-channel blockers (CCBs), reduce proteinuria by preventing the constriction of both afferent and efferent arterioles.
- Some CCBs just affect afferent arterioles, which makes proteinuria worse.
- In the treatment of proteinuria, new medications like efonidipine and benidipine are used with ARBs and ACE inhibitors.
- By inhibiting the renin-angiotensin-aldosterone pathway, the immunosuppressive and down-regulating effects of vitamin D and its analogs reduce proteinuria.
Dietary Management
- High sodium consumption might cause an increase in the glomerular capillary pressure. A diet low in salt is recommended for those with fluid excess and nephrotic syndrome.
- Most people just require a no added salt diet, while others may require limitations of as little as 40 mmol/day.
- Eating less protein can help to reduce urinary protein excretion.
- Eating more fiber and regular exercise may reduce cholesterol and maintain blood sugar.
Complications of Proteinuria
- Hypertension
- Chronic kidney disease
- Coronary heart disease, heart attack
- Gastrointestinal hemorrhage
- Cerebrovascular disease (e.g., stroke)
- Hypercholesterolemia (in case of nephrotic syndrome)
- Pulmonary edema
- Hypercoagulability (Due to loss of clotting factors)
- Venous thromboembolism
- Infections (due to loss of immunoglobulins)
Prevention of Proteinuria
Screening is advised since early detection and therapy are linked to better results in terms of decreased morbidity and mortality in proteinuria. Adults should be screened for one or more of the risk factors listed below:
- Chronic Kidney Disease
- Hypertension
- Diabetes
- Obesity
- Family history of renal disease
- Cardiovascular disease
Prognosis
- Early detection and care of proteinuria patients improve their prognosis.
- Proteinuria is used to predict the prognosis of a variety of disorders. It is related to a poorer patient outcome in IgA nephropathy.
- Similarly, in individuals with chronic renal disease, increased proteinuria is associated with a bad prognosis.
- It is also associated with a poor outcome in idiopathic membranous nephropathy.
- Proteinuria after renal transplantation is related to increased mortality and decreased graft survival.
- Proteinuria in a preeclamptic patient predicts poor results for both the mother and the baby.
Summary
- Proteinuria is defined as more than 150 mg of urine protein excretion per day. It can be caused by a variety of factors, some of which are benign (e.g., fever, strenuous activity, dehydration) and others of which are more severe (e.g., glomerulonephritis, multiple myeloma).
- It can be caused by one of two pathophysiological mechanisms: injury to the glomeruli (glomerular), damage to the tubules (tubular), or overproduction of low-molecular-weight proteins (overflow).
- If proteinuria is found, patients should be investigated further (e.g., further urinalyses) to discover the etiology.
- The treatment is determined by the underlying cause and may include drugs to lower blood pressure or manage an underlying ailment, dietary and activity adjustments, and, in certain circumstances, dialysis or kidney transplant.
- Frequent renal function testing is essential for treating proteinuria and avoiding problems.
References
- Bökenkamp, A. (2020). Proteinuria—take a closer look! Pediatric Nephrology, 35(4), 533-541. https://link.springer.com/article/10.1007/s00467-019-04454-w
- Carroll, M. F., & Temte, J. L. (2000). Proteinuria in adults: a diagnostic approach. American family physician, 62(6), 1333-1340. https://www.aafp.org/pubs/afp/issues/2000/0915/p1333.html
- D’amico, G., & Bazzi, C. (2003). Pathophysiology of proteinuria. Kidney international, 63(3), 809-825. https://doi.org/10.1046/j.1523-1755.2003.00840.x
- Grauer, G. F. (2011). Proteinuria: measurement and interpretation. Topics in companion animal medicine, 26(3), 121-127. https://doi.org/10.1053/j.tcam.2011.04.002
- Haider, M. Z., & Aslam, A. (2022). Proteinuria. StarPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK564390/
- Lamb, E. J., MacKenzie, F., & Stevens, P. E. (2009). How should proteinuria be detected and measured?. Annals of clinical biochemistry, 46(3), 205-217. https://journals.sagepub.com/doi/pdf/10.1258/acb.2009.009007
- Thomas, B. (2021, Dec 14). Proteinuria. Medscape. Retrieved on 2023, April 17 from https://emedicine.medscape.com/article/238158-overview
- Viswanathan, G., & Upadhyay, A. (2011). Assessment of proteinuria. Advances in chronic kidney disease, 18(4), 243-248. https://doi.org/10.1053/j.ackd.2011.03.002
- Zhang, A., & Huang, S. (2012). Progress in pathogenesis of proteinuria. International journal of nephrology, 2012. https://doi.org/10.1155/2012/314251