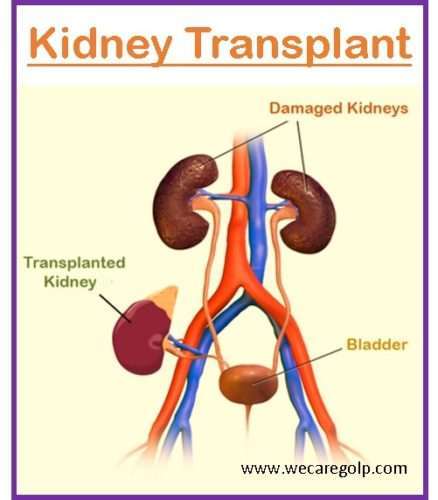
Introduction
A kidney (renal) transplant is a surgical procedure that replaces a damaged kidney with a healthy kidney obtained from a donor. The best replacement therapy for people with end-stage renal disease (ESRD) is a kidney transplant since it extends lifespan and quality of life.
- However, the number of available suitable donor organs is low.
- The transplantation rate is less than 20% of patients in need per year.
- When deciding whether to include a patient on the waiting list, it is essential to clarify whether the patient can expect a long-term improvement in their situation due to the transplantation or whether the transplantation represents too high a risk for the patient.
- Most adult patients with ESRD are never referred for transplant assessment and have a 70% 5-year mortality rate on dialysis.
- After transplantation, age-related mortality rates are highest in the first year and vary from 6.8% for those over 50 to 2% for those between 18 and 34.
- Acute reversible rejection can occasionally occur after several months of good function, especially if the patient does not take the immunosuppressive drugs as prescribed.
- Transplantation often enables most patients to resume an improved lifestyle and life expectancy compared to dialysis patients.
Advantages of Kidney Transplant
- It is the optimal therapy for end-stage renal disease (ESRD)
- In addition, transplant recipients frequently have a higher quality of life and survival rate. Recipients have a 10-year survival advantage over dialysis recipients.
- Renal transplantation has become a more cost-effective option compared to hemodialysis and peritoneal dialysis due to significant advances in early graft survival and long-term graft function.
- It reduces long-term mortality rates in ESRD patients.
Indications of Kidney Transplant
- ESRD (end-stage renal disease):
- Irreversible glomerular filtration rate (GFR) drops below 15 mL/min/1.73 m2
- Serum creatinine level of greater than 8 mg/dl)
- Chronic Kidney Disease (CKD): As a patient’s CKD progresses to stage 4, which is indicated by a GFR of less than 30 mL/min/1.73 m2, they should consult a nephrologist and get information regarding kidney failure and available treatments, including transplantation.
- Renal cell carcinoma
- Wilms tumor
- Diabetes mellitus– Diabetic nephropathy
- Hypertensive kidney disease
- Polycystic Kidney Disease
- CKD/ESRD causes:
- Prerenal (chronic or acute ischemia)
- Intrinsic renal (glomerulonephritis, focal-segmental glomerulosclerosis)
- Postrenal (reflux nephropathy, obstruction).
- Congenital disorders leading to ESRD
- Renal dysplasia
- Renal hypoplasia
- A combined kidney-pancreas transplant may be an option for patients with type 1 Diabetes and chronic renal disease who have not responded to conventional therapy.
- Nephrotic syndrome
- Interstitial nephritis
Contraindications of Kidney Transplant
Absolute contraindications
- Metastatic cancer, Active malignancy
- Active drug or alcohol addiction
- Untreated malignancy, malignant melanoma within the prior 5 years
- Serious cardiac or peripheral vascular disease
- Active or chronic infection (e.g., tuberculosis, HIV)
- Non-sanitized or systemic tumor disease
- Uncontrolled psychiatric disease
Relative contraindications
- Chronic liver disease
- Co-morbidities: uncontrolled diabetes mellitus, hypertension
- Morbid obesity with a recommended body mass index (BMI) of less than 40 kg/m2
- History of medication and dialysis non-compliance
- Frailty
- Structural genitourinary tract anomaly
- Active gastric or duodenal ulcers
- Psychiatric problems (inability to cope)
- Limited life expectancy (defined as less than the anticipated waiting time for a kidney)
- Infections like Hepatitis B and Hepatitis C
Recipient Selection
- The majority of ESRD patients have several co-morbidities and problems as a result of their renal condition. As a result, patients are thoroughly examined for their capacity to endure surgery and the immunosuppression that follows transplant surgery. Every candidate must go through a comprehensive risk-versus-benefit analysis before being approved for transplantation.
- Infection (HIV, Hepatitis B or C, tuberculosis), neoplasm, and correctable coronary artery disease diagnosis should all be regular components of the candidate workup. Candidates with diabetes and those who are older have more mortality rate than other transplant recipients.
- Existence of a possibly damaging antibody against the donor’s kidney at the time of the planned transplant is one of a few absolute immunologic contraindications to transplantation.
- Natural antibodies against ABO blood type antigens, as well as antibodies against human leukocyte antigen (HLA) Class I (A, B, C) or Class II (DR) antigens, are detrimental and can result in graft failure very early. These antibodies are regularly eliminated by screening for ABO incompatibility in the candidate, HLA typing of the donor and recipient, and direct cross-matching of the candidate serum with donor lymphocytes.
Criteria for kidney transplant
- Have a five-year life expectancy with a successful transplant
- Be in sufficient physical and nutritional condition to withstand the transplant
- Progressive, irreversible renal disease
- No active malignancy or infection
- No systemic disease, which severely restricts rehabilitation
- Effective family or social support systems
- Willingness to adhere to requirements for treatment and follow-up
- Psychologically fit
Donor Selection
Kidneys can be donated by either live or deceased donors, with deceased donors accounting for most renal transplants.
Living Donation
- A kidney can be donated from
- A living blood relative, such as a parent or a sibling
- A living non-relative, such as a spouse or very close friend
- Living-donor transplants account for up to 30% of all kidney transplants performed as a laparoscopic donor nephrectomy, whether related or unrelated (rarely this is done as an open nephrectomy in modern practice).
- It is important for live volunteer donors to be in excellent health and to share the same major ABO blood type since swapping major blood groups lowers the chance that the allograft will survive. Nonetheless, a recipient who is an A, B, or AB can have a kidney transplant from a type “O” donor. Family members that share some HLA antigen compatibility are frequently selected as donors.
- Selective renal arteriography should be performed on donors to rule out the existence of many or abnormal renal arteries because this condition complicates surgery and prolongs the ischemia time for the transplanted kidney. Donors should be honest about their health since there is a possibility that the recipient will get hepatitis, HIV, or a malignant tumor.
- Living donor eligibility criteria:
- Ages 18 to 70 years
- BMI less than 35 kg/m
- No active malignancy
- No active infection
- Adequate kidney function (GFR > 80).
- Living donor contraindications:
- BMI greater than 40 kg/m
- Diabetes
- Active malignancy
- HIV infection
- Drug addiction
- Sepsis with proven multi-resistant germs
- GFR <70 mL/min/1.72m
- Albuminuria
- Hypertension (Currently on at least one medicine)
- Pelvic or horseshoe kidneys
- Psychiatric disorders
Deceased (Post-mortem) Donation
- Donation after Brainstem Death (DBD) or Donation after Circulatory Death (DCD) are classified as a deceased donor.
- DBD transplant patients have a one-year survival rate of about 97%, whereas living donor transplant recipients have a one-year survival rate of around 99%.
- Donors who have successfully passed a formal brain death test are considered to be brain dead.
- Patients who are designated as DCD donors are those who, although not meeting the requirements for formal brain death, are thought by neurologists to be unlikely to make a significant recovery from their neurologic condition.
- The typical criteria for a deceased kidney donor are
- Already registered as an organ donor or consent from relatives after the death
- Proven loss of brain function
- Under the age of 70
- Exhibits no signs of irreversible renal failure
- Has no known risk factors for disease transmission to the recipient
- Has no known communicable illness or cancer
- When there is a risk of disease transmission, the kidney may be used with the recipient’s permission.
Procedure of Kidney Transplant
Preoperative Preparation
Before being placed on the kidney transplant waiting list, patients often had a thorough medical and surgical evaluation to detect serious comorbidities that would exclude transplantation.
Preliminary Examinations Relevant to Transplantation
Basic investigations of the patients may take from more than three months before transplantation to the operation day.
Medical history
- Patient´s history
- Family history
Clinical examination
- Cardiological and radiological examinations:
- Chest X-ray
- Electrocardiogram (ECG)
- Pulmonary function test
- Coronary angiography in patients over 50 years of age and diabetics
- Doppler sonography (head, pelvic, leg vessels)
- If necessary, stress echocardiography/myocardial scintigraphy.
- Urological examination
- Ultrasonography (USG)
- Prostate examination (men ≥ 45 years)
- Utero cystoscopy if necessary
- Bladder manometry if necessary
- Investigations to rule out foci of inflammation
- Dental examination
- X-ray of paranasal sinus and ENT examination
- USG of the upper abdomen
- Gastroscopy if necessary
- Gynecological examination for women
- Laboratory tests
- HIV
- CMV-IgG, IgM
- Herpes
- Epstein-Barr-Virus-AK
- Hepatitis-B-Antigen, Hepatitis-C-Antigen, or Hepatitis antibody
- Candida-AK
- Urine status, urine culture
- Blood grouping
- Anticoagulating factors: Prothrombin time (PT), partial thromboplastin time (PTT)
- Parathormone (PTH)
- HbA1c (for diabetes patients)
- Creatinine, Urea
- Complete blood count, Differential blood count
- Electrolytes: NA, K, Ca, Cl, inorganic P
- Bilirubin, GOT, GPT, γ-GT, AP
- Cholesterol, Triglyceride
- Amylase, Lipase
- Total protein, Electrophoresis
- Blood sugar
- Tumor markers
- Renal function test (RFT)
- Liver function test (LFT)
Other Preparation
- Informed consent from the patient as well as from relatives.
- The patient should be nil per oral prior 6 hours before surgery.
- The patient will be evaluated psychologically to determine their preparedness for transplant surgery and to discover any issues that may interfere with their recovery.
- Several drugs should be safely stopped in patients with severe renal disease at the time of transplantation including
- Most antihypertensive medications
- Phosphate binders
- Cinacalcet
- Erythropoiesis-stimulating agents (ESAs)
- Potential transplant candidates who are anticoagulated with warfarin require immediate anticoagulation reversal before surgery.
- Patients with type 2 diabetes should refrain from taking hypoglycemic medication throughout the preoperative fasting period, with routine capillary glucose measurement every 1-2 hours. To avoid ketoacidosis, patients with type 1 diabetes should begin an intravenous insulin infusion shortly after admission to the hospital.
- An immunosuppressive regimen is chosen once the choice to continue with a transplant has been made. This regimen is frequently started before the recipient goes into surgery to reduce immune function before donor antigen exposure following allograft reperfusion. The immunosuppressive regimen chosen is tailored to the recipient’s circumstances, particularly his or her sense of immunological risk.
- Immunosuppression therapy must be managed against the increased risk of infection. Because ESRD patients are often admitted to hospitals, prophylaxis in patients colonized with multi-resistant pathogens should be given special concern. Commonly used immunosuppressants are
- Azathioprine (Imuran)
- Corticosteroids (prednisone
- Cyclosporine
- OKT-3 (a monoclonal antibody)
- One major reason for morbidity following transplantation is viral infections. The best timing for vaccination is just before transplantation (at least six weeks before). According to prior immunization records, all possible transplant patients should have had vaccinations before surgery.
- Tdap (Tetanus, Diphtheria, and Pertussis)
- IPV (inactivated poliovirus)
- Hepatitis B
- Meningococcal (conjugate),
- Pneumococcal (conjugate and/or polysaccharide)
- Influenza (recommended every year)
- MMRV (Mumps, Measles, Rubella, and varicella)
- Administration of prophylactic antibiotics:
- Cefazolin 1 gram IV on call to OR and 1 gram every 12 hours for three doses.
- Clindamycin 600 mg IV on call to the OR and 600 mg IV every eight hours for 24 hours if the patient has a penicillin allergy.
During Procedure
Transplant surgery always entails two surgeries, one for the donor and one for the receiver. The procedure can be done minimally invasively or, less usually nowadays, with open surgery for the living donor.
Donor surgery
- Both DCD (donation after circulatory death) and DBD (donation after brain death) organ retrievals use cold perfusion to extract the organs similarly. Moreover, a period of dissection is included in DBD retrievals to allow for the evaluation of the organs while they are being obtained.
- To reduce the exposure of the organs to heated ischemia, quick cannulation of the iliac artery with cold perfusion is carried out during DCD retrieval.
- The colon is mobilized to gain full abdominal exposure and access to the retroperitoneal area. Heparinization of the donor is followed by the identification and isolation of the arteries and ureter(s).
- The kidneys are then removed together with the ureter, renal vein, and artery using patches of the IVC and aorta. The organs are subsequently brought to the rear table for further perfusion and evaluation.
- Nephrectomy for living donor kidney transplantation is most frequently carried out using a laparoscopic approach. The left kidney is favored since it has a longer renal vein, but in these situations, no IVC or aorta patch may be obtained. Once the kidney has been removed, it should once again be flushed with a preservation fluid.
For organ preservation
- Once the kidneys have been obtained, they must be kept in perfusion fluid (preservation solution).
- The kidney is rinsed with an approx. 4 degrees Celsius cold perfusion solution and surface cooling, e.g., with ice water.
- The aim is to reduce intracellular metabolic processes, among other things, by cooling down (hypothermia) with special perfusion solutions and thus lowering the energy consumption of the cells.
- With the perfusion of the organs, the cold ischemia time (preservation time) begins, which should currently not be longer than 36 hours.
- However, the primary aim is to keep the preservation time as short as possible.
- Warm ischemia is traditionally referred to as the “sew-in” period, which begins when the organ is taken from cold storage and ends when it is reperfused following vascular anastomosis.
- After a macroscopic examination of the kidney, it is packed individually in a sterile bag and stored on ice in transport boxes.
- Anatomical features and medical data of the donor are noted in a corresponding organ report.
- If the placement decision has already been made, the kidney will be transported to the recipient center.
Recipient surgery
- If the kidney is brought in from another facility, it will be received frozen and preserved in perfusion fluid.
- Before the extracted kidney will be implanted in the recipient, the following steps should be done.
- Conservation of the ureter length properly
- Removal of any extra surrounding fat
- Identification of the renal artery and vein
- Flushing the kidney with preservation solution
- Correction of any leakage
- In the iliac fossa, most frequently on the right side, the graft is positioned extraperitoneally. The iliac vessels are revealed by dissecting retroperitoneally in the iliac fossa, and any detected lymphatics are bound.
- Between the donor renal vein and the recipient’s external iliac vein, as well as between the donor renal artery and the recipient’s internal or external iliac artery, termino-lateral anastomoses are created.
- A ureteroneocystostomy is formed, the kidney is reperfused, and the ureter is anastomosed to the bladder. A ureteric stent, which may be removed around six weeks after the transplant, is placed over the area to be anastomosed.
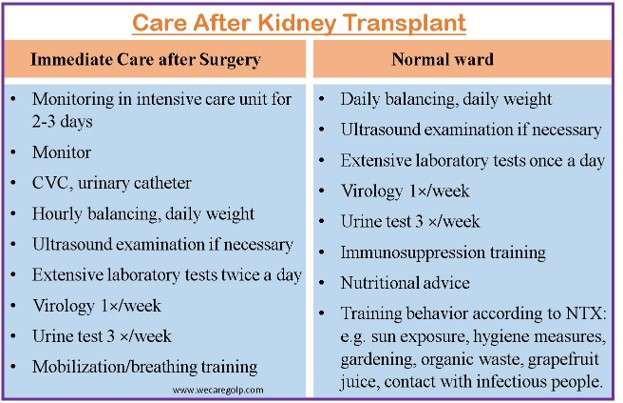
After Procedure
- The patient is closely monitored in the initial postoperative phase.
- The initial objective is to control the dynamic fluid balance of a fresh kidney, which can respond to a high urea nitrogen load with an osmotic diuresis but has little capacity for urine concentration or salt reabsorption.
- Fluid balance must be maintained, hypertension treatment may need adjustment, and electrolyte imbalances may need to be corrected while kidney function improves.
- The following tests and procedures are part of the standard postoperative regimen: Each day, blood pressure, weight, urine output, and blood pressure are monitored. Serum creatinine (SCr) testing is a daily lab investigation to be carried out after transplantation.
- After transplantation, prophylactic antibiotic therapy is continued for 24 to 36 hours.
- Sips of water may be allowed before increasing the diet as tolerated after the digestive function has been restored.
- The patient begins with a clear fluid diet, continues to full fluids, and then, as soon as it is tolerated, advances to solid food. Daily total fluid intake must be adjusted against the patient’s volume status.
- The amount of urine produced is initially noted hourly. The catheter often remains in place for two to five days at the very least. Before the Foley catheter is taken out, a retrograde cystogram may be performed.
- In the case of an uncomplicated kidney transplant, the patient usually stays in the hospital for four days. After stabilization, the patient is discharged to the transplant outpatient clinic for follow-up care.
Drugs used in Kidney Transplant
Induction therapy
- Monoclonal antibodies:
- Muromonab-CD3
- Basiliximab
- DaclizumaB
- Alemtuzumab
- Polyclonal antibodies:
- Antithymocyte globulin
Maintenance therapy
- Calcineurin inhibitors (CNI)
- Cyclosporine
- Tacrolimus
- Purine synthesis inhibitors/APA
- Azathioprine
- Mycophenolate mofetil (MMF)
- Steroids
- Prednisone
- mTOR inhibitors
- Sirolimus
- Everolimus
Complications of Kidney Transplant
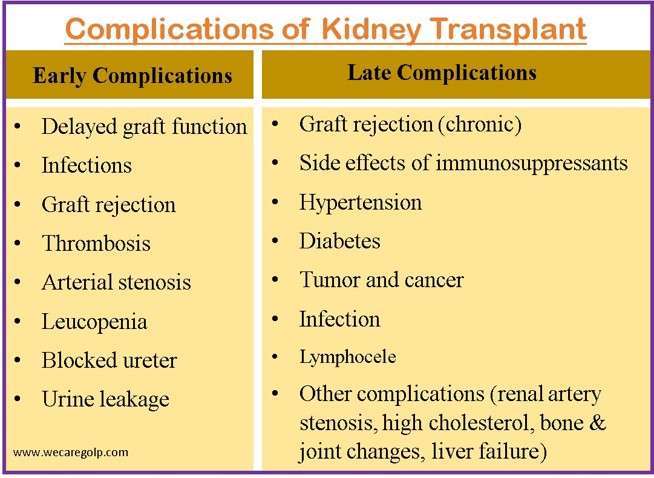
Early Complications
Delayed graft function
- Delayed graft function (DGF) is defined as the inability of the kidney transplant to function promptly, necessitating dialysis in the first post-transplantation week.
- While the patient is generally oliguric, non-oliguric renal dysfunction may emerge.
- When a transplanted kidney fails to function, it is vital to rule out arterial or venous occlusion, as well as urine blockage or leak.
- An urgent ultrasound with Doppler to measure renal flow determines this.
- Individuals with surgical issues may require an emergency reoperation.
- Causes may include
- Acute tubular necrosis (ATN)
- Acute rejection
- Thrombotic microangiopathy (TMA)
- Obstruction
- Thrombosis
- Poor initial kidney function
- Recurrent primary renal disease
Infection
- Kidney-transplanted patients have an increased susceptibility to infection due to immunosuppression.
- Infection remains a significant cause of morbidity and death after transplantation, even though, the use of preventive antibiotic medication at the time of surgery has significantly reduced these risks.
- Within the first three months following the transplant, up to 30% of kidney transplant recipients get infected.
- Early detection and adequate treatment are crucial.
Infections within 1 month after transplantation
- Infections with resistant pathogens
- VRE
- MRSA (Methicillin-resistant staphylococcus aureus)
- Candida-Species
- Other relevant complications
- Wound infection
- Catheter infection
- Anastomosis insufficiency
- Aspergillus infection
- Pseudomonal infection
- Urinary tract infection
- Cold and flu
- Rarely, there are possibilities to obtain infections from the organ donor
- Rabies
- West-Nil-Virus
- HIV
Infections between 1 to 6 months after transplantation
- Influenza
- BK virus
- Herpes simplex stomatitis (HSV)
- Cytomegalovirus (CMV)
- Pneumonitis
- Hepatitis (B, C)
- Adenovirus
- Esophageal gastroenteritis
Graft rejection
- Episodes of graft rejection happen throughout the first 26 weeks following transplantation. It is crucial to keep in mind that most rejection incidents are reversible. Rejection typically has no symptoms.
- Typically, an increase in serum creatinine signals the presence of rejection, and a kidney biopsy is used to confirm the diagnosis.
Types of graft rejection in the early phase
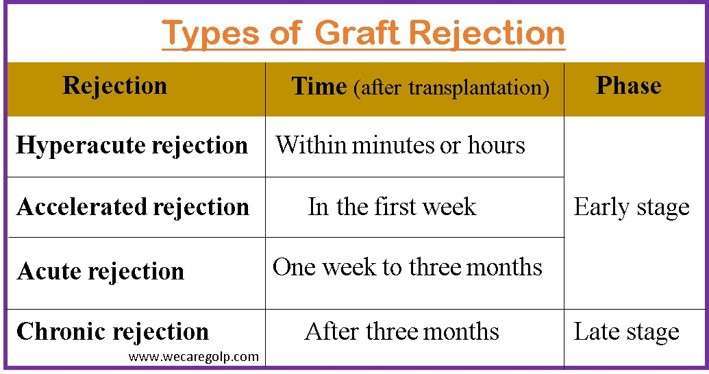
Hyperacute rejection
- When the transplant recipient already has antibodies to antigens in the donor’s kidney, hyperacute rejection may happen.
- It happens minutes or hours after transplantation and causes irreversible damage.
- Yet, hyperacute rejection is extremely uncommon when using current cross-matching methods.
Accelerated rejection
- The presence of pre-existing antibodies in the transplant recipient causes accelerated rejection.
- That happens in the first week after surgery.
- Usually, it results from the recipient’s antibodies attacking the kidney donated.
- These antibodies were probably existing before the transplant but at levels below those that could be detected.
Acute rejection
- One week to three months following the transplant, acute rejection is most frequently observed.
- The reaction is mostly cell-mediated.
- The level of serum creatinine rises as a result.
- Since it is uncommon, other symptoms might include
- Decreased urine production,
- Fever,
- A swollen and sensitive graft, and
- Edema of the lower extremity on the transplanted side.
Thrombosis
- Although very uncommon, renal vein thrombosis carries a substantial risk of transplant loss.
- In the early postoperative phase, this phenomenon may show up as
- New-onset hematuria,
- Abrupt-onset oliguria/anuria, and/or
- Graft failure.
Arterial stenosis
- This consequence frequently has no symptoms, but blood pressure may rise.
- When graft function is compromised, ultrasonography evaluation frequently leads to its identification (elevated serum creatinine).
- Angiography can be used for transluminal angioplasty and is both diagnostic and therapeutic.
Leucopenia
- After a kidney transplant, low white blood cell (WBC) counts are typically observed.
- It is normally linked to medication.
- The drugs that are most frequently involved include mycophenolate, azathioprine, and valganciclovir.
- Low WBC is also a side effect of cotrimoxazole and several other drugs.
Blocked ureter
- After a kidney transplant, the ureter, which transports urine to the bladder, may become blocked.
- It can be blocked by
- Blood clots (soon after the transplant)
- Scar tissue (in the late stage).
- A blocked ureter can be fixed with surgery or by draining it with a catheter.
Urine leakage
- After surgery, the urine may occasionally leak from the junction of the ureter and bladder.
- It typically occurs within the first month following the procedure.
- The fluid may leak through the surgical incision.
- In most cases, additional surgery may need to fix a urine leak.
Late Complications
Chronic graft rejection
- Often, it appears after three months after the transplant.
- The transplanted kidney has progressively stopped working as a result of repeated immune system assaults on the allogeneic major histocompatibility complex (allo-MHC) produced by its component cells.
- It increases patient morbidity and mortality and increases the likelihood of allograft loss.
Side effects of immunosuppressive drugs
- An increased risk of infections
- Hypertension
- Diabetes
- Infection
- Hair loss or hair growth
- Diarrhea
- Weight gain
- Cancer (especially skin cancer)
- Bleeding or bruising
- Thinning of bones
Hypertension
- A kidney transplant frequently results in long-term complications such as high blood pressure.
- It might be because of
- Certain kinds of immunosuppressive medications
- Some transplanted patients may have a risk of developing hypertension since the pre-transplantation period.
- Usually, high blood pressure does not show any symptoms, but it can make it more likely to get other serious conditions like heart disease, stroke, and heart attacks.
Diabetes
- Diabetes is a common complication of renal transplant.
- It may be due to
- Some types of immunosuppressive drugs
- Eating more as patients feel well.
Tumor and cancer
- Virus-causing cancer may be developed due to the long-term use of immunosuppressive medicines after a kidney transplant like
- Melanoma and non-melanoma (skin cancer)
- Lymphoma (lymphatic system cancer)
- Kaposi’s sarcoma (skin and internal organs cancer)
- Kidney cancer
- Cervical cancer
- Avoiding sun exposure and applying sun scream to exposed areas may prevent the risk of skin cancer to some extent.
Infection
There is also a risk of getting an infection after 6 months after transplantation.
- Pneumonia
- UTI (urinary tract infection)
- Infections with Aspergillus, atypical mole, Mucor species
- CMV infection
- Hepatitis (HBV and HCV) HSV-Encephalitis, SARS, West-Nil-Virus
Lymphocele
- The accompanying lymphatics are damaged when the iliac vessels are exposed, which leads to this problem.
- When doing this dissection, lymphatic tissue should be ligated carefully whenever feasible.
- Patients could exhibit swelling and soreness above the transplanted kidney.
- As an alternative, the collection might infect the graft, compress it, and impair its function.
- Percutaneous drainage is used to treat symptomatic lymphoceles.
Other complications
- High cholesterol
- Chronic liver failure
- Bone and joint changes
- Pyelonephritis
- Renal artery stenosis
Summary
- A kidney transplant is a surgical procedure in which a healthy kidney from a donor is substituted for a dysfunctional or failing kidney. Patients with end-stage renal disease who need or are expected to soon need dialysis often undergo the surgery.
- A live or deceased donor, usually a family member or acquaintance, can provide the donor’s kidney. A healthy kidney is surgically implanted into the patient’s abdomen, usually without removing the damaged kidney.
- Immunosuppressive drugs are necessary for patients after the transplant to prevent the immune system from rejecting the new kidney. Patients must be monitored closely for side effects including infection and organ rejection since these drugs have negative effects.
- Compared to dialysis, kidney transplantation can provide patients with several advantages, including better quality of life and a longer lifetime.
- It has some risks, though, and to guarantee the best results, a detailed assessment of the patient and potential donors is required.
References
- Abramyan, S., & Hanlon, M. (2021). Kidney Transplantation. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK567755/
- Collins, B.H. (2021, Dec 17). Kidney Transplantation. Medscape. Retrieved on 2023, April 1 from https://emedicine.medscape.com/article/430128-overview
- Danovitch, G. M. (2012). Handbook of kidney transplantation. Lippincott Williams & Wilkins. https://books.google.com.np/books?id=pdgPAAAAQBAJ&lpg=PR7&ots=E7tPKWSu86&dq=kidney%20transplant%20&lr&pg=PA43#v=onepage&q=kidney%20transplant&f=false
- Garcia, G. G., Harden, P., & Chapman, J. (2012). The global role of kidney transplantation. Kidney and Blood Pressure Research, 35(5), 299-304. https://doi.org/10.1159/000337044
- Humar, A., & Matas, A. J. (2005, Nov). Surgical complications after kidney transplantation. In Seminars in dialysis, 18(6), pp. 505-510. Malden, USA: Blackwell Science Inc. https://doi.org/10.1111/j.1525-139X.2005.00097.x
- Karuthu, S., & Blumberg, E. A. (2012). Common infections in kidney transplant recipients. Clinical Journal of the American Society of Nephrology, 7(12), 2058-2070. Doi: 10.2215/CJN.04410512
- Kasiske, B. L., Zeier, M. G., Chapman, J. R., Craig, J. C., Ekberg, H., Garvey, C. A., … & Balk, E. M. (2010). KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney international, 77(4), 299-311.doi: 10.1097/TP.0000000000003136
- Morris, P., & Knechtle, S. J. (2008). Kidney transplantation: principles and practice. Elsevier Health Sciences. https://books.google.com.np/books?id=QufZDud4y6IC&lpg=PT44&ots=KgR5AYnG67&dq=kidney%20transplantation%20&lr&pg=PT44#v=onepage&q=kidney%20transplantation&f=false
- Naik, R. H., & Shawar, S. H. (2022). Renal transplantation rejection. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK553074/
- TeachMeSurgery. (2021). Renal Transplantation. Retrieved on 2023, April 1 from https://teachmesurgery.com/transplant-surgery/organ-transplantation/renal/