Dialysate (Dialysis Solution for the Hemodialysis)
Dialysate, also called dialysis fluid or dialysis solution, is a simple composition of pure treated water, an acidic component (acid) and a base component (sodium bicarbonate). It is mixed in the dialysis machine before it comes to the dialyzer.
- The electrolyte and acid-base imbalances are typical effects of end-stage renal failure.
- It can compensate relatively well, even temporarily and partially, according to an individual’s need during the hemodialysis.
- On the semi-permeable membrane of the dialyzer, the diffusion process is dependent on the difference in concentration of a substance between the blood plasma and the dialysis solution and is basically possible in both directions.
- For example, uremia toxins and potassium remove, while bicarbonate and calcium pass to the blood plasma.
- The counter-principle maintains the concentration difference across the dialyzer.
- The convection process (passive entrainment of a substance during ultrafiltration) is also possible in both directions (filtration and back filtration).
Importance of Dialysate
- The composition of the dialysis solution is essential for correcting or stabilizing the electrolyte and acid-base balance.
The Components of Dialysate
- The Dialysis Water
- The Acid Concentrate
- The Base Components
The Dialysis Water
- Advance and modern water treatment make it possible to provide every patient the ultra-pure dialysis water.
- The goal of water treatment for dialysis is to remove as many dissolved and undissolved substances as possible from the drinking water.
- A combination of filtration, ion exchange, and reverse osmosis is used to produce dialysis water.
- Water treatment for dialysis includes all components that are required in a dialysis center for the production and distribution of the dialysis water from the entrance to the building to the dialysis machines.
The Acid Concentrate
- Due to the diffusion of dissolved substances from the blood into the dialysis solution and vice versa, the blood concentrations can be increased, decreased, or kept constant.
- The acidic component is available in canisters (and containers for central supply systems) as a dissolved liquid.
- CO2 gas is constantly escaping from a canister of dialysis solution. The amount of the escaping gas depends on the storage time, the air permeability of the canister and the ambient temperature. As a result, the pH increases and the acid concentration in the canister steadily decreases. Therefore, if possible, bicarbonate canisters should always be used up within one day of treatment and tightly sealed.
- The acid concentrate of the bicarbonate dialysis contains 2 – 7 mmol/l acetate. It helps the pH adjustment regarding CO2 loss.
- Click “The Acid Concentrate in Bicarbonate Dialysis to read more!
Sodium
- Sodium significantly influences the volume and osmolarity of the extracellular fluid. Therefore, sodium is vital in maintaining a stable circulation during hemodialysis.
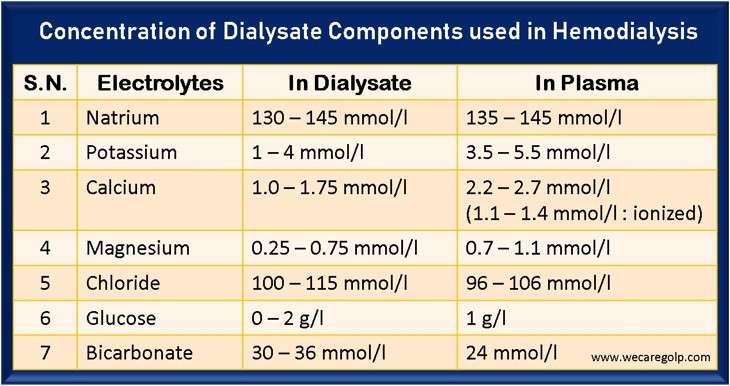
- The average value of plasma sodium concentration is between 135 – 145 mmol/l
- It is readily dialyzable.
- The usual sodium concentration in the dialysis solution is 130 – 145 mmol/l.
- When selecting the sodium concentration in the dialysis solution, the patient’s blood pressure, water retention and thirst must consider.
- Essentially, Sodium removal during dialysis depends on the principle of ultrafiltration.
Potassium
- The average value of the plasma potassium concentration is between 3.5 – 5.5 mmol/l.
- The usual potassium concentration in the dialysis solution is 1 – 4 mmol/l
- Potassium levels drop most rapidly during the first hour of dialysis because the highest potassium concentration is in the cells.
- The rapid drop in extracellular potassium can trigger cardiac arrhythmia.
- In insulin-dependent diabetics, it must remember that insulin causes glucose and potassium to be absorbed into the cell, thus worsening potassium removal during dialysis.
- However, correctly used glucose-containing dialysis solution prevents hypoglycemia in people with diabetes.
- But potassium removal could increase by up to 25% with a glucose-free dialysis solution.
- If the blood pH is too low (acidosis), hydrogen ions enter the cell, and potassium flows out of the cell into the extracellular space to compensate.
- During dialysis, the supply of bicarbonate leads to blood pH normalization and a return flow of potassium into the cells.
- That´s why regular blood gas analysis is also mandatory to prevent hyperkalemia.
Calcium
- The average concentration of calcium in the serum is about 2.2 – 2.7 mmol/l.
- Half of the total calcium measured in serum is albumin-bound. 10% is present as complex salts (lactate, citrate, bicarbonate), and the rest (40%) in the ionized form (1.1 – 1.4 mmol/l).
- During hemodialysis, the calcium in the dialysate of 1.0 – 1.75 mmol/l is matched only by the ionized portion of the serum calcium. It means that calcium transport predominantly diffuses from the dialysis solution into the blood.
- Calcium is very easily dialyzable.
Magnesium
- The average level of magnesium in serum is about 0.7 – 1.1 mmol/l.
- Magnesium levels in dialysis patients generally elevate due to the lack of renal elimination.
- Correction of this elevation by the dialysis solution is expected to positively affect the lifespan of the erythrocytes and improve nerve conduction velocity.
- The usual magnesium concentration in the dialysis solution is 0.5 – 1.0 mmol/l.
Chloride
- Sodium, potassium, calcium, and magnesium are predominantly bound to chlorine in the blood.
- The average value of chloride is between 96-106 mmol/l.
- Chloride and bicarbonate change inversely to each other, i.e., the decrease of bicarbonate leads to hyperchloremia and vice versa.
- Usually, the chloride concentration in the dialysis solution is 100 – 115 mmol/l.
Glucose
- Glucose in the dialysis solution prevents blood sugar losses during dialysis, leading to complications (hypoglycemia), especially in people with diabetes.
- The glucose values in the plasma of a healthy individual are around one g/l.
- Glucose can diffuse easily through the dialysis membrane.
- Thus, when using a dialysis solution with a glucose concentration of 1 g/l, there is no significant glucose exchange between blood and dialysate.
- If, on the other hand, the glucose concentration in the dialysis solution is 2 g/l, the blood absorbs approx. 15-30 g of glucose per dialysis corresponds to an energy intake of about 300-400 kcal.
- Today, dialysis is usually performed with 1-2 g/L (100 – 200 mg%) or glucose-free.
The Base Components (Buffer Substances)
- Due to terminal renal failure, the acid-base balance in the blood is disturbed.
- The result is increasing acidosis.
- During dialysis, acid-base balance can restore by eliminating H+ ions on the one hand and substituting a buffer substance (acetate/bicarbonate) on the other.
- The buffer substance diffuses from the dialysis solution into the patient’s blood. Here, the buffer substance concentration in dialysis solution must be higher than in the blood.
Acetate
- In the past, acetate was used as a buffer system primarily for technical reasons for hemodialysis.
- The acetate concentration was 35 – 40 mmol/l in the dialysis solution. Only one concentrate uses for acetate dialysis.
- One bicarbonate molecule neutralizes one acetate molecule, with one hydrogen ion consumed per molecule.
Disadvantages of Acetate
- Since bicarbonate diffuses from the blood into the dialysis solution following the concentration gradient and the metabolism of acetate in the body takes a certain amount of time, patients initially become more acidic at the beginning of acetate dialysis.
- Acidosis leads to the additional release of potassium from the cells into the plasma.
- In children and muscle-deficient patients with low metabolic capacity, “acetate stasis” may occur.
- This causes low blood pressure (hypotension) due to peripheral vasodilation and nausea.
- Furthermore, vomiting and headache are not uncommon during acetate dialysis.
- Finally, acetate leads to hypoxemia (decreased respiratory drive due to CO2 loss) and cytokine production, thus is “bioincompatible.”
Bicarbonate
- Two concentrates are used in bicarbonate dialysis: the so-called acid component and a bicarbonate solution (8.4%).
- As the base component, dry bicarbonate concentrates have become established in bags (Bibag) and cartridges (Bicart). Because of lighter and smaller than canisters, these reduce the transport costs and storage requirements.
- Bicarbonate is the most important physiological buffer substance. The average standard value for healthy people is around 24 mmol/l.
- On the other hand, the dialysis solution usually contains 32 mmol/l (30 – 36 mmol/l) bicarbonate so that bicarbonate is supplied to the patient. The goal of treatment is mild alkalosis at the end of dialysis and blood gas parameters as close as possible to average values at the beginning of the subsequent dialysis.
- Depending on the concentration gradient, bicarbonate diffuses from the dialysis solution into the patient’s blood from the start of dialysis to correct the metabolic acidosis.
- The K/DOQI guidelines recommend a pre-dialytic bicarbonate value for the patient of 22 mmol/l.
- In practice, bicarbonate concentrations of 30 – 36 mmol/l are set in the dialysate.
- Click “bicarbonate in bicarbonate dialyse” to read more!
References
- https://www.sciencedirect.com/topics/nursing-and-health-professions/dialysate
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4495470/
- Michael V. Rocco (2012): Nephrology Secrets: Hemodialysis. USA, Philadelphia: ELSEVIER MOSBY, third edition; 52:372.
- https://www.davita.com/treatment-services/dialysis/in-center-hemodialysis/what-is-hemodialysis