The Acid Concentrate in Bicarbonate Dialysis
The acid concentrate is vital in bicarbonate dialysis. Due to the diffusion of dissolved substances from the blood into the dialysis solution and vice versa, the blood concentrations can be increased, decreased, or kept constant.
- The substances like sodium and magnesium should only correct slightly up or down.
- Electrolytes, such as calcium and potassium, can be adjusted to the diverging needs of the patient through an individual composition of the acidic component.
- Substances that should always remove from the blood, such as phosphate, are not included in the dialysis solution.
- The acidic component is available in canisters (and containers for central supply systems) as a dissolved liquid.
Sodium
Sodium significantly influences the volume and osmolarity of the extracellular fluid. Therefore, sodium is vital in maintaining a stable circulation during hemodialysis.
- 98% of body sodium is in the extracellular space, 2% in the intracellular area. Active transport mechanisms prevent sodium from entering the cell. In this way, it keeps water bound extracellularly.
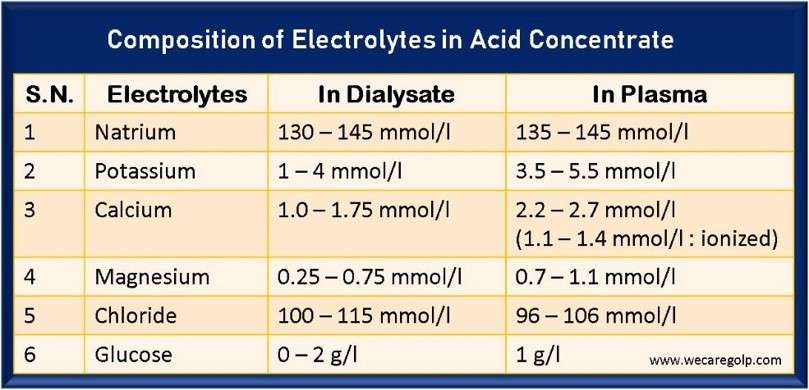
- The average value of plasma sodium concentration is between 135 – 145 mmol/l
- Total body sodium is approximately 95 grams (4100 mmol).
- Due to its low atomic weight (23), sodium is readily dialyzable.
Sodium in dialysis solution
- When selecting the sodium concentration in the dialysis solution, the patient’s blood pressure, water retention and thirst must consider.
- Essentially, Sodium removal during dialysis depends on the principle of ultrafiltration.
- The protein-free ultrafiltrate contains approximately the same sodium concentration as the plasma. High ultrafiltration rates also lead to increased sodium removal. About 6.9 grams of sodium chloride remove per liter of ultrafiltrate.
- To avoid major shifts in osmolality during dialysis, the sodium concentration of the dialysis fluid should be approximately equal to that of the plasma.
- But, if one wants to prevent diffusive sodium withdrawal from the serum, the sodium concentration in the dialysis solution should be at least 3 mmol/l higher than the serum sodium concentration (Gibbs-Donnan effect).
Dialysis-induced hyponatremia:
- Ultrafiltration removes sodium as an important osmotically active substance from the extracellular space.
- In addition, diffusive loss of sodium occurs due to a low sodium concentration in the dialysis solution.
- At the same time, fluid migrates from the extracellular space into the intracellular space (osmosis).
- The result is an osmotic Disequilibrium Syndrome with the symptoms of muscle cramps, headache, nausea, and vomiting.
- The more severe the dialysis-induced hyponatremia, the more pronounced the symptoms: seizures, coma, hemolysis.
Dialysis-induced hypernatremia:
- High sodium levels in the dialysis-free interval raise the patient’s blood pressure.
- In severe hypernatremia (serum sodium > 150 mmol/l), water transfers from the intracellular space to the extracellular space.
- The result is intracellular (especially cerebral) dehydration with the following symptoms: headache, nausea, vomiting, heat waves, disorientation, seizures, spasticity, and coma.
- The infusion of 100 ml of physiological saline (0.9%) means the application of 0.9 grams of sodium chloride. The injection of 10 percent saline corresponds to administering 1 gram of sodium chloride. If the administration is done at the end of dialysis (as is often the case), the sodium chloride remains in the body.
Potassium
- A constant potassium concentration is essential for maintaining the vital function of the body’s cells.
- The total body potassium is 3,800 mmol. Only 2% localizes in the extracellular space and 98% locates in the intracellular space. That´s why any cell death (hemolysis, large hematomas, gastric bleeding) can cause threatening hyperkalemia in obligate dialysis patients.
- The average value of the plasma potassium concentration is between 3.5 – 5.5 mmol/l
- With food, 50 – 100 mmol of potassium is absorbed daily.
- 90 – 95% of this excretes by the healthy kidneys, 5 – 10% is lost through the intestines, and 1 – 5% through sweating.
- Since potassium excretion via the intestines can increase with increasing renal insufficiency, a tendency to hyperkalemia is only expected with oliguria (below 500 ml urine).
- Diarrhea and diuretic intake eliminate more potassium (cause hypokalemia).
Potassium concentration in the dialysis solution
- Potassium is removed 85% diffusively and 15% convectively during dialysis.
- Potassium levels drop most rapidly during the first hour of dialysis because the highest potassium concentration is in the cells.
- To be removed by dialysis, it must migrate from the intracellular space into the extracellular space and then into the intravascular space.
- The rapid drop in extracellular potassium can trigger cardiac arrhythmia.
- Potassium value does not correspond approximately to the actual conditions during or directly after dialysis.
- After dialysis, the measured serum potassium increases by about 30% due to the rebound effect.
- That means potassium moves from the intracellular space into the extracellular space.
- In insulin-dependent diabetics, it must remember that insulin causes glucose and potassium to be absorbed into the cell, thus worsening potassium removal during dialysis.
- However, correctly used glucose-containing dialysis solution prevents hypoglycemia in people with diabetes.
- But potassium removal could increase by up to 25% with a glucose-free dialysis solution.
- If the blood pH is too low (acidosis), hydrogen ions enter the cell, and potassium flows out of the cell into the extracellular space to compensate.
- During dialysis, the supply of bicarbonate leads to blood pH normalization and a return flow of potassium into the cells.
- That´s why regular blood gas analysis is also mandatory to prevent hyperkalemia.
- As a rule, a change in blood pH of 0.1 leads to an opposite potassium shift of 0.4 – 0.5 mmol/l
Hypokalemia
- Potassium below 3.5 mmol/l can be as dangerous for a patient as hyperkalemia.
- If low normal or decreased potassium values are repeatedly measured in a dialysis patient without significant residual excretion, the cause must investigate.
- Hypokalemia before the start of dialysis mainly occurs with vomiting, diarrhea, laxative abuse, diuretic therapy with residual excretion still present, and potassium-free parenteral nutrition.
- Dialysis-induced hypokalemia at the end of treatment is due to diffusive potassium transfer from the blood to the dialysate.
- Suppose the serum potassium value before dialysis is within the normal range (=4.5 mmol/l); the potassium concentration in the dialysate should be increased from the standard 2 mmol/l to 3-4 mmol/l.
- Hypokalemia can lead to severe cardiac arrhythmias in the form of tachyarrhythmias and even ventricular fibrillation, particularly in patients with cardiac diseases (especially those taking long-term digitalis preparations) and in the presence of additional electrolyte or acid-base disorders.
Hyperkalemia
- Due to dietary errors, hyperkalemia occurs in many dialysis patients during the dialysis-free interval.
- The metabolic acidosis further exacerbates hyperkalemia by diffusing H+ ions into the cell and, in return, potassium passing out of the cell into the intravascular space (blood).
- The symptoms of hyperkalemia include paresthesia, confusion states, and muscular weakness, which can progress to paralysis.
- With serum potassium levels above 6.5 mmol/l, the heart’s electrical activity disorder and higher degree AV-blocks with bradycardia can occur.
- The ECG shows wide and plump ventricular complexes, high and peaked T waves, and prolonged PQ time.
- If predialytic potassium is high (>6.5 mmol/l), it is reasonable to also start dialysis with a high concentration of potassium (e.g., 4 mmol/l) in the dialysate and then decrease it by 1 mmol/l every hour during dialysis.
Calcium
- The human organism contains approx. 1000 – 1200 grams of calcium.
- Of this, 99% is found in the bone tissue and the teeth, the rest in the intra- and extracellular space.
- Calcium is not only the main mineral component of bones but also of great importance as an intracellular messenger for many physiological processes.
- The average concentration of calcium in the serum is about 2.2 – 2.7 mmol/l.
- Half of the total calcium measured in serum is albumin-bound. 10% is present as complex salts (lactate, citrate, bicarbonate), and the rest (40%) in the ionized form (1.1 – 1.4 mmol/l).
- Ionized calcium is the biologically active form of calcium.
- The ionized calcium concentration depends on the pH value and the albumin concentration.
- Acidosis increases and alkalosis decreases the ionized fraction.
- Protein deficiency decreases the protein-bound fraction and relatively decreases the ionized fraction (and vice versa).
Calcium in the dialysis solution
- During hemodialysis, the calcium in the dialysate of 1.0 – 1.75 mmol/l is matched only by the ionized portion of the serum calcium. It means that calcium transport predominantly diffuses from the dialysis solution into the blood.
- Patients with terminal renal insufficiency often show hypoalbuminemia because of malnutrition, which leads to hypocalcemia in the total serum calcium. However, the ionized calcium is within the normal range.
- It is, therefore, advisable in nephrology to correct the total calcium to an albumin value of 4 g/dl or a total protein value of 7.76 g/dl for better comparability with the reference range.
- Calcium is very easily dialyzable.
- In addition to diffusive mass transport, a considerable convective transport of substances (ultrafiltration) takes place with calcium.
- A patient-specific selection of the calcium concentration in the dialysis solution of 1.25 – 1.75 mmol/l, considering the osteological situation of the patient, the prescribed phosphate binders, and the administration of active vitamin D, can lead to a balanced calcium level.
- When prescribing calcium-containing phosphate binders, the calcium concentration in the dialysis solution tends to be selected lower (1.25 mmol/l), considering the patient’s compliance.
- Calcium also has a positive inotropic effect and raises blood pressure by increasing heart rate. Therefore, a “high-calcium” dialysis solution for hypertensive patients and a “low-calcium” dialysis solution for heart failure patients are less suitable.
Magnesium
- Magnesium is essential for normal bone formation and the transport and utilization of metabolic fuels (fat, amino acids).
- It influences the potassium permeability of the cell membrane and enables cells to maintain high intracellular potassium concentrations.
- In pharmacological doses, magnesium lowers peripheral vascular resistance and thus blood pressure.
- The average level of magnesium in serum is about 0.7 – 1.1 mmol/l.
- Magnesium deficiency is associated with cardiac arrhythmia and disturbances in glucose metabolism.
- High magnesium levels may also have a negative effect on lipid metabolism. On the other hand, they are associated with lower diastolic blood pressure values and slow the progression of arteriosclerosis, which is somewhat contradictory.
- An elevated magnesium level inhibits the parathyroid glands and the conversion of amorphous calcium phosphate into hydroxyapatite, i.e., the bone maturation process.
- Magnesium levels in dialysis patients generally elevate due to the lack of renal elimination.
- Correction of this elevation by the dialysis solution is expected to positively affect the lifespan of the erythrocytes and improve nerve conduction velocity.
- The usual magnesium concentration in the dialysis solution is 0.5 – 1.0 mmol/l.
- In the case of adynamic renal osteodystrophy, reducing the magnesium content to 0.25 mmol/L in dialysis solution may suggest in individual cases.
Chloride
- Sodium, potassium, calcium, and magnesium are predominantly bound to chlorine in the blood.
- Together with one or two chlorine ions, they form a chloride, e.g., sodium chloride (common salt).
- In chronic renal failure, serum chlorides are usually slightly elevated (97-108 mmol/l). Normalization is not necessarily sought.
- However, it results from selecting an optimal buffer concentration in the dialysis solution. Chloride and bicarbonate change inversely to each other, i.e., the decrease of bicarbonate leads to hyperchloremia and vice versa.
- Usually, the chloride concentration in the dialysis solution is 100 – 115 mmol/l.
Glucose
- Glucose in the dialysis solution prevents blood sugar losses during hemodialysis, leading to complications (hypoglycemia), especially in people with diabetes.
- Additionally, glucose can use as a caloric supplement in cases of patient with malnutrition or consumptive disease.
- The glucose values in the plasma of a healthy individual are around one g/l.
- With a molecular weight of 180 Daltons, glucose can diffuse easily through the dialysis membrane.
- Thus, when using a dialysis solution with a glucose concentration of 1 g/l, there is no significant glucose exchange between blood and dialysate.
- If, on the other hand, the glucose concentration in the dialysis solution is 2 g/l, the blood absorbs approx. 15-30 g of glucose per dialysis corresponds to an energy intake of about 300-400 kcal.
- Today, dialysis is usually performed with 1-2 g/L (100 – 200 mg%) or glucose-free.
Glucose-free Dialysis
- With a glucose-free dialysis solution, 25-30 g of glucose losses into the dialysate; with 2g of glucose in the dialysis solution, about 20 g of glucose adds to the body.
- Glucose-free dialysis solution is problematic in diabetics, children, the elderly, and the severely ill because of the risk of severe hypoglycemia.
- Post dialytic fatigue syndrome and EEG changes seem to occur more frequently.
- Protein catabolism may also increase.
- On the other hand, potassium removal is greater by about 25% with glucose-free dialysate since lower reactive insulin levels lead to potassium leakage from the cells.
- In contrast, there are possible effects of glucose intake on lipid metabolism with triglyceride elevation, but these become detectable only at higher glucose concentrations in the dialysate (400-500 mg%).
- A concentration of 1 g/L (100 mg) glucose in the dialysis fluid can be recommended.
References
- Flanigan M.J.: Sodium flux and dialysate sodium in hemodialysis. Seminar in Dialysis (1998) 11/5: 298. https://doi.org/10.1111/j.1525-139X.1998.tb00372.x
- Peticlerc T. Et al: Electrolyte modeling: Sodium. Is dialysate sodium profiling actually useful? Nephrol Dial Transplant (1996) 11 (Suppl. 2): 35-38
- Flanigan M.J. er al.: Dialysate sodium delivery can aler chronic blood pressure management. Am J Kidney Dis (1997) 29/3: 383-391
- Mann H., S. Stiller: Urea, sodium, and water changes in profiling dialysis. NDT (1997) 11 (Suppl. 8): 10-15
- Blumberg A. et al.: Plasma potassium in patients with terminal renal failure during and after haemodialysis; relationship with dialytic potassium removal and total body potassium. NDT (1997) 12: 1629-1634
- Redaelli B.: Electrolyte monitoring in haemodialysis – potassium. NDT (1996) 11 (Suppl. 2): 39-41
- Dolson G.M., H.J. Adrogoue: Low dialysate K+ decreases efficiency of hemodialysis and increases urea rebound. J Am Soc Nephrol (1998) 9: 2124-2128
- Argiles I Ciscart A.: Points to remember when selecting dialysate calcium concentration. NDT (1995) 10: 451-454
- Avis L. et al.: An optimal potassium, calcium, and magnesium dialysate for general use? Dialysis and Transplantation (1996) 25/6: 354-358
- Navaro-Gonzales J.F.: Magnesium in dialysis patients: serum levels and clinical implications. Clinical Nephrology (1998) 49/6: 373-378
- Weinreich T.: Hypermagnesämie oder Magnesiummangel? Nieren- und Hochdruckkrh. (1997) 26 (Suppl. 1): 86-90
- Rosborough D.C., J.C. Van Stone: Dialysate Glucose. Seminars in Dialysis (1993) 6/4: 260-263
- Basics of base in hemodialysis solution: Dialysate buffer production, delivery and decontamination – PMC (nih.gov)