Bicarbonate in Bicarbonate Dialysis
There is a significant role of bicarbonate in bicarbonate dialysis. Two concentrates are used in bicarbonate dialysis: the so-called acid component and a bicarbonate solution (8.4%).
Bicarbonate Buffer
- Hydrogen ions (H+) formed during metabolism are buffered to form carbonic acid (H2CO3), which decomposes into carbon dioxide (CO2) and water (H2O). Carbon dioxide exhales. In renal insufficiency, the direct excretion of hydrogen ions and the reabsorption of bicarbonate (HCO3) in the kidney impair, and metabolic acidosis occurs.
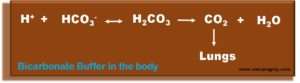
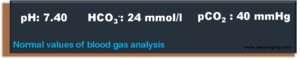
- Bicarbonate is the most important physiological buffer substance. The average standard value for healthy people is around 24 mmol/l.
- On the other hand, the dialysis solution (aslo called dialysate) usually contains 32 mmol/l (30 – 36 mmol/l) bicarbonate so that bicarbonate is supplied to the patient. The goal of treatment is mild alkalosis at the end of dialysis and blood gas parameters as close as possible to average values at the beginning of the subsequent hemodialysis.
Bicarbonate and dialysis solution
In the dialysate, the following aspects must observe when setting the bicarbonate concentration (mmol/l or conductivity in mS):
- CO2 gas is constantly escaping from a canister of bicarbonate solution. The amount of the escaping gas depends on the storage time, the air permeability of the canister and the ambient temperature. As a result, the pH increases and the bicarbonate concentration in the canister steadily decreases. Therefore, if possible, bicarbonate canisters should always use up within one day of treatment and tightly seal.
- The buffer differs from patient to patient and from dialysis to dialysis. A high-protein diet or an infection can temporarily increase the buffer requirement. A slight increase in fluids in the dialysis-free interval improves the acid-base balance and vice versa. Regular blood gas analysis is essential.
- Depending on the concentration gradient, bicarbonate diffuses from the dialysate into the patient’s blood from the start of dialysis to correct the metabolic acidosis. On the other hand, bicarbonate is lost in the same concentration as it is present in the blood due to convective mass transport during ultrafiltration. These complex processes are almost impossible to quantify.
- The K/DOQI guidelines recommend a pre-dialytic bicarbonate value for the patient of 22 mmol/l.
- In practice, bicarbonate concentrations of 30 – 36 mmol/l are set in the dialysate. Bicarbonate concentrations below 30 mmol/l in the dialysate are not sufficient to ensure acceptable predialytic serum bicarbonate values until the subsequent dialysis.
- The acid concentrate of the bicarbonate dialysis contains 2 – 7 mmol/l acetate. It helps the pH adjustment regarding CO2 loss. Even these low acetate concentrations can, under certain circumstances, cause side effects in sensitive patients.
- Standard blood gas analyzers calculate the bicarbonate concentration in the blood based on the measured carbon dioxide partial pressure (pCO2) according to the Henderson-Hasselbalch equation.
Calcification and Acetate
- The conductivity measuring electrodes are very sensitive to calcification.
- Calcifications are the result of chemical reactions of the dialysate.
- An incorrect or too high bicarbonate concentration leads to pH values exceeding 7.5 in the dialysate.
- When this occurs, the bicarbonate ions will dissociate, i.e., the bicarbonate breaks down into H ions and carbonate ions.

- The carbonate releases in this way immediately and forms a bond with the divalent ions magnesium and calcium. This bond is no longer reversible.
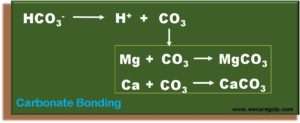
- Even a minimal concentration of carbonate ions can be enough for this reaction to take place in seconds.
- Once the reaction has started, it will continue even if the pH corrects again. But the process will slow down to some extent.
- To reduce or prevent this reaction, the acidic component of bicarbonate dialysis contains a small amount of acetate (2-3 mmol/l).
- The acetate neutralizes the same amount of bicarbonate, producing carbonic acid (H2CO3). Carbonic acid again decomposes into carbon dioxide (CO2) and water.

- This buffer system stabilizes the bicarbonate-containing dialysis solution by binding CO2 and minimizes chemical reactions and calcification.
References
- https://www.sciencedirect.com/topics/nursing-and-health-professions/dialysate
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4495470/
- Michael V. Rocco (2012): Nephrology Secrets: Hemodialysis. USA, Philadelphia: ELSEVIER MOSBY, third edition; 52:372.
