Introduction
A cervical biopsy is a minor surgical procedure in which a small amount of cervical tissue is removed for examination to detect abnormal or precancerous conditions or cervical cancer.
- A cervical biopsy may be performed when cervical abnormalities are found during a pelvic examination or abnormal cells are found during a pap test.
- Precancerous cells are cells that appear to be abnormal but are not cancerous.
- The abnormal cells may be the first sign of cancer that may develop later.
- A colposcopy, also known as a colposcopy-guided cervical biopsy, frequently includes a cervical biopsy.
- Cervical biopsies are slightly more invasive than Pap tests or colposcopies.
Epidemiology
- Over 80% of cervical cancer is diagnosed at an advanced clinical stage, which often has a very poor diagnosis.
- By 2016, the majority of labs (67.2%) in the College of American Pathologists Pap Education Program had implemented the 2014 updated Bethesda System (TBS), and 20.1% had plans to do so.
- By early 2003, 85.5% of laboratories in the United States had implemented Bethesda 2001 terminology and the adoption of TBS in the international cytopathology community had produced a significant impact.
Indications of Cervical Biopsy
Histopathological examination of tissue through colposcopy-guided biopsy is the standard method for diagnosing cervical precancerous lesions. A cervical biopsy is performed to confirm the diagnosis of cancer when abnormalities are found. In the following situations, a cervical biopsy is necessary:
- Abnormal Pap smear test
- Positive Human Papillomavirus (HPV) test
- Cervical polyps
- Genital warts
- Diethylstilbestrol (DES) exposure
- Post-coital bleeding
- Abnormal menstrual bleeding
- Bleeding after menopause
- Irregular or heavy menstrual bleeding
- Suspected signs of cancer
Types of Cervical Biopsy
Colposcopic (punch) biopsy
- A colposcopy, also known as a colposcopy-guided cervical biopsy, frequently includes a cervical biopsy. Colposcope is an instrument that allows visualization of the vagina and cervix.
- This procedure uses a circular blade, like a paper hole puncher, to remove a sample tissue.
- Biopsy is taken from the suspected area or a four-quadrant using punch biopsy forceps.
- It is done on an outpatient basis without anesthesia.
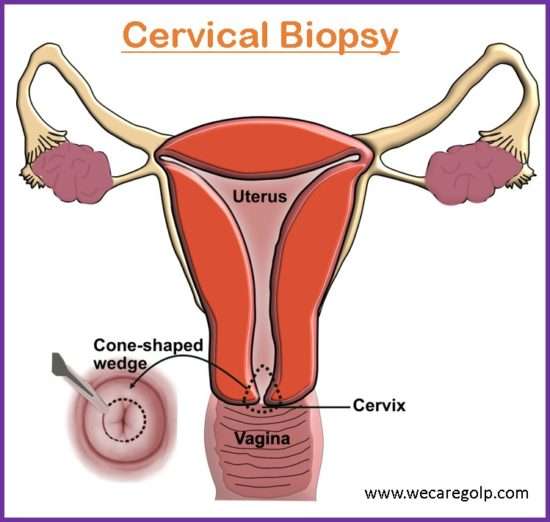
Cone biopsy
- Conization is another name for a cone biopsy.
- Both diagnostic and therapeutic applications are possible with it.
- A large cone-shaped piece of tissue from the cervix is removed during this surgical procedure.
- Method for removal of a cone may be a cold knife, CO2, laser, or laser diathermy loop.
- The cone-shaped tissue may contain an entire squamous-columnar junction, stroma with gland, and endocervical mucous membrane.
Indications for a cone biopsy
- Diagnostic and therapeutic purposes in cervical intraepithelial neoplasia (CIN)
- Unsatisfactory and inconsistent colposcopic findings
- Complete removal of precancerous tissues
- Treatment of early-stage cancer
Endocervical curettage (ECC)
- This procedure uses a narrow instrument called a curette to scrape the lining of the endocervical canal.
- The curette is then gently scraped against the walls of the cervical canal, removing some of the tissue.
- It is done when the transformation zone is not visible with a colposcopy.
- Endocervical scraping is another name for it.
Wedge biopsy
- It is done when definite growth is visible.
- The ideal site for biopsy is an area near the edge.
- Under this biopsy, after the insertion of a vaginal speculum, the anterior and posterior lip of the cervix is held by alley’s forceps.
- With a scalpel, a wedge of tissues is cut from the edge of the lesion including the healthy tissue for comparative histological study.
Ring biopsy
- It is the removal of the whole squamocolumnar junction area of the cervix with a special knife.
Articles required for Cervical Biopsy
- Kidney tray
- Bowels 2
- Vaginal speculum
- Acetic acid solution
- Allis forcep
- Sterile Gloves
- Lubricant
- Cystoscope
- Povidone 10% solution
- Normal saline
- Cotton balls/swabs
- Lidocaine spray/lignocaine gel
- Endocervical curette
- Cytobrush
- Dissolvable suture material
- Needle drivers
- Scissors
- Forceps
- Labelled specimen jar containing 10% formalin
- Sanitary pads for the patient after the procedure.
Procedure of Cervical Biopsy
Before the procedure
- Refrain from using any vaginal creams, medications, or powders in, or around the vagina for 24 to 48 hours.
- Avoid vaginal intercourse or tampons for 24-48 hours before and after the procedure.
- Get informed consent.
- Explain the procedure to the patient and family members.
- Allow the patient to empty the bladder.
During the procedure
- Maintain privacy.
- Place the patient in a dorsal recumbent position.
- Perform vaginal examination.
- Insert vaginal speculum of appropriate size.
- Clean and soak the cervix with acetic acid.
- A colposcope is used to visualize the vagina.
- Allis forcep is used to hold the cervix for biopsy.
- The type of biopsy depends on the shape, size, and location of the abnormal cells. Depending on the type of cervical biopsy, the process of removal of cervical tissue differs.
- One or more small samples of tissue will be taken using a special type of forceps called an endocervical curette or an endocervical brush.
- For a cone biopsy, a loop electrosurgical excision procedure (LEEP) or the cold knife cone procedure may be done.
- Bleeding from the biopsy site is treated with electrocauterization or suturing.
- Send the tissue to the lab for testing.
- Recording and reporting.
After the procedure
- Collect the sample in a container containing 10% formalin.
- Proper naming of the specimen should be done.
- Send the specimen to the lab with proper documentation.
- Check the vital signs.
- Check the vaginal bleeding.
- Put the patient on sanitary pads and check for heavy bleeding.
- Pain management.
- Keep the women on bed rest for 24 to 48 hours.
After Discharge from the hospital
- Do not allow the patient to douche, use tampons, or have sex for 1 week after the biopsy or as per the health care provider’s suggestion.
- Notify the physician if the woman has any of the following symptoms:
- Bleeding
- Foul-smelling discharge from the vagina
- Fever
- Severe lower abdominal pain
Side Effects after Cervical Biopsy Procedure
- Pain
- Fever/infection
- Heavy bleeding
- Foul-smelling vaginal discharge
- Psychological distress
The Bethesda System
- The Bethesda System (TBS) is the standardized reporting system in cervicovaginal cytology. TBS reports elements including specimen type, specimen adequacy, general categorization, interpretation, or result.
- The Bethesda system was first introduced in 1998 and revised in 1991, 2001, and 2014.
- The World Health Organization (WHO), the American Society for Colposcopy and Cervical Pathology (ASCCP), and the College of American Pathologists now recommend the two-tier classification of the squamous intraepithelial lesion. (the high-grade and low-grade lesions).
- The Human Papillomavirus (HPV) affects the intraepithelial essentially in two ways: either as a viral infection or viral-associated pre-cancer.
Principles
- The patient’s health care provider must receive clinically relevant information from the laboratory through terminology.
- Different pathologists and laboratories should use the same terminology, and it should also be adaptable enough to be used in a wide range of laboratory settings and locations.
- The terminology must reflect the most recent understanding of cervical neoplasia.
The 2014 Bethesda System (Cervical Biopsy Interpretations)
Specimen Type
- Indicate conventional smear (Pap smear) vs. Liquid-based preparation vs other
Specimen Adequacy
- Satisfactory for evaluation (describe the presence or absence of endocervical /transformation zone component and any other quality indicators, e.g., partially obscuring blood, inflammation, etc.)
- Unsatisfactory for evaluation… (specify reason)
- Specimen rejected/ not processed (specify reason)
- Specimen processed and examined, but unsatisfactory for evaluation of epithelial abnormality because of…. (specify reason)
- Samples are smeared directly onto a microscope slide after collection
- Liquid-based cytology
- The sample is taken from the transitional zone using an arrow-shaped brush.
- The cells are collected in a bottle of preservative and transported to the laboratory.
General Categorization (optional)
- Negative for intraepithelial lesion or malignancy
- Epithelial cell abnormality
- Other
Interpretation/Result
Negative for intraepithelial lesion or malignancy
(When there is no cellular evidence of neoplasia, state this in the General Categorization above and/or in the Interpretation/Result section of the report-whether there are organisms or other non-neoplastic findings).
Non-neoplastic Findings
- Non-neoplastic cellular variations
- Squamous metaplasia
- Keratotic changes
- Tubal metaplasia
- Atrophy
- Pregnancy-associated changes
- Reactive cellular changes associated with
- Inflammation (includes typical repair)
- Radiation
- Intrauterine contraceptive device
- Glandular cells status post hysterectomy
Organisms
- Trichomonas vaginalis
- Fungal organisms morphologically consistent with Candida species
- Shift in flora suggestive of bacterial vaginosis
- Bacteria morphologically consistent with Actinomyces sp.
- Cellular changes consistent with herpes simplex virus
- Cellular changes consistent with cytomegalovirus
Other non-neoplastic findings
- Endometrial cells (in a woman ≥ 45 years age)
Specify if negative for squamous intraepithelial lesion)
Epithelial cell abnormalities
Squamous cell abnormalities
- Atypical squamous cells
- Of undetermined significance
- Cannot include High-grade squamous intraepithelial lesion (HSIL)
- Low-grade squamous intraepithelial lesion (LSIL): Encompassing: HPV/mild dysplasia/CIN1
- High-grade squamous intraepithelial lesion (HSIL): Encompassing moderate and severe dysplasia, CIS; CIN 2 and CIN 3
- with features suspicious for invasion
- Squamous cell carcinoma
Glandular cell
- Atypical
- Endocervical cells
- Endometrial cells
- Glandular cells
- Atypical
- Endocervical cells, favor neoplastic
- Glandular cells, favor neoplastic
- Endocervical adenocarcinoma in situ
- Adenocarcinoma
- Endocervical
- Endometrial
- Extrauterine
- No otherwise specified
Other malignant neoplasms (specify)
Adjunctive testing
- Report the test’s outcome with a brief explanation that the clinician can easily understand.
Computer-assisted interpretation of cervical cytology
- If an automated device examines a case, specify the device and the result.
Educational notes and comments appended to cytology reports (optional)
- Suggestions should be concise and consistent with clinical follow-up guidelines published by professional organizations (references to relevant publications may be included).
Benefits
- It leads to the early detection and treatment of intraepithelial lesions.
- It provides effective communication among cytopathologists and referring physicians.
- It facilitates cytologic-histopathological correlation.
- It provides reliable data for national and international statistical analysis comparisons.
Contraindications of Cervical Biopsy
- Active cervical and vaginal infection
- Incompetent cervix
- Pelvic pain
- Heavy vaginal bleeding
- Injury to cervical tissue
- Cervical stenosis
- Late pregnancy or active labor
- If a patient does not consent to a cervical biopsy
Complications of Cervical Biopsy
- Secondary hemorrhage
- Cervical stenosis
- Infertility
- Cervical incompetence
- Mid-trimester abortion or preterm labor
Summary
- A cervical biopsy is a surgical procedure in which a small amount of tissue is removed from the cervix, often using a colposcope.
- Indications of a cervical biopsy are abnormal pap smear tests, cervical polyps, genital warts, positive HPV test, post-coital bleeding, etc.
- Depending on the location, extent, and severity of the cervical lesion, a variety of cervical biopsy options are available.
- Furthermore, special patient preparation is necessary before, during, and after the cervical biopsy.
- The interpretations of cervical biopsy are based on the Bethesda system that classifies the cervical lesions based on the specimen type, adequacy, general category, interpretation, and adjunctive testing.
References
- Robson, J., Merwe, C., Walters, L., Noack, L., Giles, S.M. (2022). The occasional cervical biopsy. Can J Rural Med, 27 (2),72-76. https://www.cjrm.ca/text.asp?2022/27/2/72/341022
- Nayar, R., & WILBUR, C.D. (2015, March – April). The Pap Test and Bethesda 2014. Acta Cytologica, 59 (2), 121-132. https://doi.org/10.1159/000381842
- Reyes, M. C., Cooper, K. (2014, August). Cervical Cancer Biopsy Reporting. A Review. Indian J Pathol Microbiol. 57, 364-8. https://www.ijpmonline.org/text.asp?2014/57/3/364/138713
- Wang, Y,. Wang, J,. & Mei, H. (2022). Diagnosis of Cervical Intraepithelial Neoplasia and Invasive Cervical Carcinoma by Cervical Biopsy under colposcopy and Analysis of Factors Influencing. Emergency Medical International, 2022, 9621893. Doi: 10.1155/2022/9621893
- https://www.hopkinsmedicine.org
- https://www.healthline.com
- https://www.cancercenter.com
- https://www.uptodate.com