Proportioning of the Dialysis Solution
Proportioning means the defined enrichment of the dialysis solution with electrolytes.
- The electrolytes are now available as an acid concentrate in liquid form in containers, canisters, and the bicarbonate – as Bicart or Bibag.
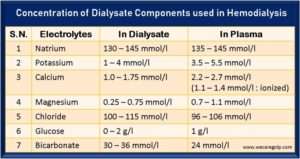
- The acid concentration usually contains five electrolytes: sodium (Na+), potassium (K+), calcium (Ca2+), magnesium (Mg2+), and chloride (Cl–); and the nonelectrolyte glucose.
- Additionally, sodium bicarbonate (NaHCO3) is a commonly used electrolyte as a base in bicarbonate dialysis.
- Dialysis solution prepares in the dialysis machine immediately before it is used in the dialyzer.
- The treated pure water comes from the reverse osmosis system to the treatment station via a ring line, at the same time, the concentrated electrolyte solution via a central concentrate system, or concentrate canisters.
- Freshly prepared dialysis solution circulates continuously to the dialyzer in the extracorporeal circuit. The effluent dialysate goes to the drain after making a single pass through the dialyzer.
There are two different systems of dialysate proportioning.
Volumetrically controlled proportioning
- Volumetrically controlled proportioning use on dialysis machines from Fresenius and Nipro.
- These dialysis machines have two concentrate pumps that continuously mix the bicarbonate and acid concentrate with the treated water in a specific amount.
- These concentrate pumps have a fixed stroke volume, which delivers a particular amount of concentrate per stroke.
The mixing ratio (1:34) in the basic setting between treated water and concentrates can be composed, for example, as follows:
- 32.775 parts water
- 1.225 parts sodium bicarbonate
- 1 part acid concentration.
Another commonly used mixing ratio (1:44) in the basic setting between water and concentration is as follows:
- 42.225 parts water
- 1.775 parts sodium bicarbonate
- 1 part acid concentrate.
Suppose the user changes the basic setting for the bicarbonate and sodium content in the dialysis fluid. In that case, the stroke volume of the respective concentrate pump does not change but rather the speed at which the concentrate pumps work (more or fewer strokes per unit of time).
Conductivity controlled proportioning
The dialysis machines from Gambro, B. Braun, and Nikkiso control via conductivity.
- This method first adds the bicarbonate concentrate to the water until the set bicarbonate conductivity is reached.
- A measuring cell controls the conductivity and then the concentrate pump.
- After that, the acid concentrate adds the same way until the set overall conductivity is reached.
Conductivity
- Conductivity (CD) is a measurement of the ability of the total ionic content of dialysate to conduct electricity.
- Electrolytes are substances that break down into ions in an aqueous solution.
- For example, the common salt molecule NaCl breaks down into the electrically positively charged sodium ion and the negatively charged chlorine ion. (NaCl Na+ + Cl–)
- If two electrodes are placed in a saline solution, the positively charged sodium ions (cations) migrate to the negative pole (cathode) and the negatively charged chlorine ions (anions) to the positive pole (anode).
- The faster this happens, the higher the electrical conductivity of a solution.
- The conductivity depends on the concentration of electrolytes, the temperature, and the type of electrolytes.
- Sodium chloride (NaCl) has an almost completely dissociating capacity and it is a high concentration in the dialysis solution. That´s why NaCl makes the greatest contribution to conductivity.
References
- https://patents.google.com/patent/US4360323A/en
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4495470/
- https://www.sciencedirect.com/topics/nursing-and-health-professions/dialysate
- Petitclerc, T. (2006), Do dialysate conductivity measurements provide conductivity clearance or ionic dialysance?. Kidney International: Volume 70, Issue 10, Pages 1682-1686
