Introduction
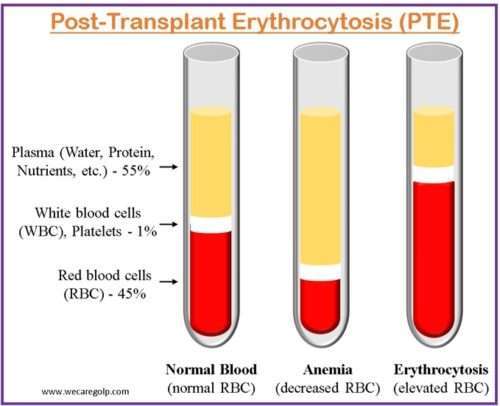
Post-transplant erythrocytosis (PTE) is defined as elevated hemoglobin (Hb) levels greater than 17 g/dL or hematocrit (Hct) levels greater than 51% for more than six months in the absence of thrombocytosis, leukocytosis, or another probable source of erythrocytosis following kidney transplantation. Erythrocytosis, also called polycythemia, is a disorder of red blood cells that occurs due to blood volume imbalances where either plasma volume decreases or red cell mass increases.
- PTE affects 8%–15% of kidney transplant recipients and develops typically 8–24 months after the surgery.
- Its consequences are unclear; however, it may raise the risk of thromboembolic events, hypertension, and cardiovascular events.
- It has long been assumed that PTE is a sign of excessive erythropoietin production, whether from an allograft or the native kidney, and will go away naturally over time.
- It is a post-transplant complication caused by excessive erythroid synthesis from the allograft plus/minus natural kidneys.
Incidence
- PTE has an estimated prevalence of 8 to 20% and is a potentially significant complication following kidney transplantation with increased morbidity and risk of thromboembolic events.
- Adult transplant recipients are more likely to develop PTE than pediatric transplant recipients.
- Male transplant recipients may be more prone to PTE than female transplant recipients.
- PTE usually appears during the first year following transplantation, however, it might appear later.
- Both live and deceased donor transplant patients can develop PTE.
Risk Factors of Post-transplant Erythrocytosis
- Male gender
- Retention of a native kidney with adequate erythropoiesis before transplant
- Transplant renal artery stenosis
- Immunosuppressive medications (cyclosporine and tacrolimus)
- Smoking
- Diabetes mellitus
- Rejection-free post-transplant course
- Shorter duration of hemodialysis before renal transplantation
- Pre-transplant renal anemia
- Hypertension
- Prior history of polycythemia vera or erythrocytosis
Signs and Symptoms of Post-transplant Erythrocytosis
- Hypertension
- Malaise
- Dizziness
- Headache
- Fatigue
- Thrombosis
- Blurred vision
- Shortness of breath
- Flushing or redness of the skin
- Thrombophlebitis
- Pulmonary embolus
Pathophysiology of Post-transplant Erythrocytosis
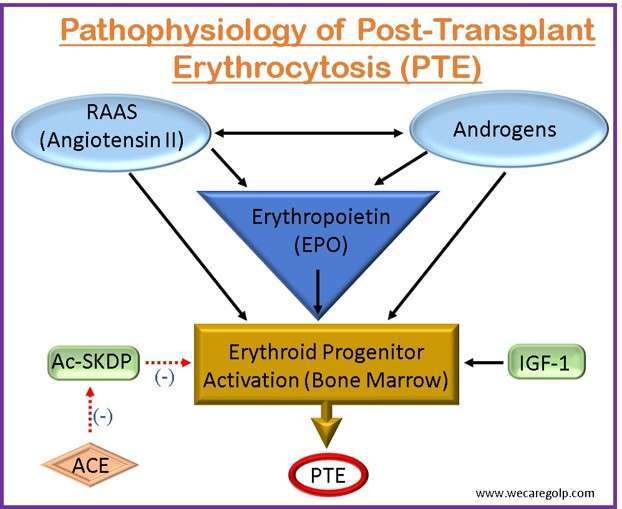
- The pathogenesis of PTE is influenced by three major hormonal systems.
- Erythropoietin (EPO)
- The renin-angiotensin-aldosterone system (RAAS)
- Endogenous androgens
- Renal hypoxia can occur after solid organ transplantation when the transplanted organ initially experiences an ischemia-reperfusion damage state. EPO, a hormone principally in charge of controlling the synthesis of red blood cells (RBCs) in the bone marrow, is stimulated by this hypoxic condition.
- The kidneys create and release more EPO into circulation in response to renal hypoxia. The bone marrow is stimulated to make more RBCs as a result of the enhanced EPO synthesis.
- The RAAS is activated during kidney transplantation as a result of renal mass loss and immunosuppressive drug usage. Angiotensin II levels rise as a result, which encourages the kidney’s synthesis of erythropoietin and encourages erythropoiesis.
- Nevertheless, persistent RAAS activation can cause erythrocytosis and excessive EPO production. This is assumed to be a result of angiotensin II’s actions on bone marrow-based erythroid progenitor cells, which increase their survival and proliferation.
- Furthermore, RAAS activation can cause hypertension and hypervolemia, both of which can promote erythropoiesis and aggravate PTE.
- To avoid organ rejection after solid organ transplantation, patients are frequently given immunosuppressive drugs. Several of these drugs, including calcineurin inhibitors like tacrolimus and cyclosporine, have been proven to boost erythropoiesis and enhance EPO synthesis.
- Modifications to renal function: The transplanted organ may eventually recover from the initial ischemia-reperfusion insult and resume normal function. The kidneys may generate less EPO as renal function increases, which can assist in restoring normal erythrocyte production.
- PTE’s pathophysiology has been linked to endogenous androgens like testosterone. Since they increase EPO synthesis and help erythroid progenitor cells survive, androgens are known to promote erythropoiesis. Men are more prone than women to acquire PTE, according to studies, which implies that androgens may be involved in the onset of PTE.
- Moreover, research has revealed that individuals who have received a kidney transplant from a male donor as opposed to a female donor are more likely to get PTE. This provides more evidence for the part that androgens play in the development of PTE.
- It is unclear how precisely androgens contribute to the development of PTE. It is likely that androgens direct or indirect signal other signaling pathways to increase EPO synthesis. On the other hand, androgens could help erythroid progenitor cells survive, increasing the generation of RBCs.
- Additionally, other erythropoiesis-stimulating factors responsible for the pathogenesis of PTE are
- The oligopeptide N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) and
- The growth factor insulin-like growth factor 1 (IGF-1)
Diagnosis of Post-transplant Erythrocytosis
- Blood test: Rise in the RBC count and/or Hb levels in a patient who has undergone a kidney transplant are typically used to make the diagnosis of post-transplant erythrocytosis. In general, PTE diagnosis is confirmed by
- Hct level more than 51% in males and 49% in women, or
- A Hb level greater than 17 g/dL in men and 16 g/dL in women.
- Imaging studies: Doppler ultrasonography or computed tomography angiography, to detect blood clots or other abnormalities in the blood vessels, are additional procedures that may be helpful in the diagnosis of PTE.
- A bone marrow biopsy may also be required to rule out further causes of erythrocytosis.
Treatment of Post-transplant Erythrocytosis
- Extracellular volume contraction (e.g., excessive diuresis, uncontrolled diabetes mellitus, diarrhea, and vomiting) must be identified and managed in patients with PTE to avoid a further rise in Hct levels.
- When Hct levels surpass 55%, intermittent venesection has typically been used to keep Hct levels between 50% and 52% to limit the risk of thromboembolic events. This technique, while helpful in correcting Hct levels, results in severe iron shortage in most patients.
- This deficit can be treated with iron supplementation, for the first three months following surgery, a patient with restored graft function may need to consume about 1 g of iron to replenish their iron reserves.
- Lowering Hb levels in individuals with PTE below 17.5 g/dl in normotensive patients is the intended therapeutic outcome. The recommendation is to continue PTE therapy indefinitely because PTE might have a relapsing course.
Medications
In transplant patients, several medications have been effective in lowering the RBC count.
- Hb levels are reduced by 0.2–0.3 g/dl in kidney transplant patients taking
- Angiotensin-converting enzyme (ACE) inhibitors and
- Angiotensin receptor blockers (ARBs).
- The use of ACE inhibitors also leads to decreased
- Ac-SDKP and
- IGF-1.
- Theophylline functions as an adenosine antagonist. Adenosine aids in the release of EPO and maybe the bone marrow response. Theophylline’s efficacy cannot be anticipated with the same accuracy as ACE inhibitors.
- Cytoreductive medicines, such as hydroxyurea and interferon alpha, can aid in the management of PTE by reducing erythropoiesis. These medications are normally reserved for patients with severe PTE who have not responded to previous treatment choices or who are unable to withstand phlebotomy. Nevertheless, the use of cytoreductive medicines may result in adverse effects such as bone marrow suppression and immunosuppression.
Renal artery embolization
- During this treatment, a catheter is inserted into the renal artery and tiny particles are injected to obstruct the kidney’s blood supply.
- Reducing erythropoietin production, which encourages the creation of RBCs, is the aim of renal artery embolization.
- Most patients handle this minimally invasive technique successfully. Yet, there is a chance of renal injury as well as other issues.
Nephrectomy
- Nephrectomy is often only performed on patients with life-threatening problems who have not responded to conventional therapies, such as severe hypertension or thrombosis.
- Nephrectomy does, however, come with some serious dangers, such as the possibility of bleeding, infection, and kidney failure.
Apheresis
- A process called apheresis or plasmapheresis entails drawing blood from the patient and separating the plasma from the RBCs.
- The plasma is subsequently discarded while the RBCs are given back to the patient.
- Apheresis can aid with PTE symptom management and Hct reduction. The results of apheresis are transient, though, and the process must be repeated often.
Phlebotomy
- Phlebotomy is the removal of blood from the body, which lowers Hct and RBC mass.
- Patients with severe PTE and Hct values exceeding 54% (for men) or 50% (for women) often qualify for it.
- Phlebotomy’s goal is to improve the Hct level to the desired range of 38-44%.
- As phlebotomy may cause severe iron deficiency, iron supplementation is necessary for those patients.
Complications of Post-transplant Erythrocytosis
Major complications
- Thromboembolism: Deep vein thrombosis, pulmonary embolism, stroke
- Hypertension
- Hyperviscosity syndrome: Headache, visual disturbances, altered mental status
- Impaired clotting mechanism
- Cardiovascular disease: Myocardial infarction
- Thrombotic microangiopathy
Minor complications
- Fatigue
- Dizziness
- Shortness of breath
- Chest pain
- Palpitations
Prevention of Post-transplant Erythrocytosis
- Patients’ erythropoietin levels, renal function, and any other conditions that may enhance the risk of developing PTE before transplantation should be monitored.
- The risk of PTE can be increased by immunosuppressive medication, which can reduce erythropoiesis. This medication should be adjusted without increasing the risk of both PTE and organ rejection.
- Hct and Hb levels should be monitored regularly during the first six months following transplantation and then at regular intervals thereafter.
- Using erythropoietin-stimulating agents (ESAs) can increase erythropoiesis and increase the risk of PTE. These medications should be avoided unless absolutely necessary.
Prognosis
- PTE has generally been associated with an increased risk of stroke and both arterial and venous thromboembolic disorder because of its nature and greater blood viscosity (such as PE, DVT, and myocardial infarction).
- With proper care, the prognosis of PTE is typically favorable. Untreated or poorly controlled PTE, on the other hand, can lead to major consequences and have a substantial influence on the patient’s quality of life. Transplant recipients must be checked for the development of PTE regularly and handled correctly to avoid problems and enhance prognosis.
- In brief, poor outcomes from post-transplant erythrocytosis have improved over time as a result of early diagnosis and better therapy with ACE-I/ARBs.
Summary
- PTE, or post-transplant erythrocytosis, can be severe and should be managed to keep Hb levels below 17.5 g/dl. PTE occurs in 8%-15% of kidney transplant recipients and has been steadily declining in recent decades.
- Most cases occur between 8 and 24 months after transplantation and manifest as moderate malaise, fatigue, or serious thromboembolic events. Men, patients without pre-transplant anemia, renal artery stenosis, patients on extensive pre-transplant dialysis courses, and stronger performing grafts have all been identified as risk factors for PTE.
- An appropriate PTE workup should involve ruling out hemoconcentration as well as secondary causes of erythrocytosis such as renal artery stenosis, hypoxic lung illness, OSA, and malignancy.
- The current standard of care is the use of ACE-I or ARB, which have been determined to be the most effective and well-tolerated therapeutic options and are also regarded to be the driving force in the decrease of PTE prevalence.
References
- Abecassis, M., Bartlett, S. T., Collins, A. J., Davis, C. L., Delmonico, F. L., Friedewald, J. J., … & Gaston, R. S. (2008). Kidney transplantation as primary therapy for end-stage renal disease: a National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQI™) conference. Clinical Journal of the American Society of Nephrology, 3(2), 471-480. Doi: 10.2215/CJN.05021107
- Alzoubi, B., Kharel, A., Machhi, R., Aziz, F., Swanson, K. J., & Parajuli, S. (2021). Post-transplant erythrocytosis after kidney transplantation: A review. World Journal of Transplantation, 11(6), 220. doi: 10.5500/wjt.v11.i6.220
- Kiberd, B. A. (2009). Post‐transplant erythrocytosis: a disappearing phenomenon?. Clinical transplantation, 23(6), 800-806. https://doi.org/10.1111/j.1399-0012.2008.00947.x
- Malyszko, J., Oberbauer, R., & Watschinger, B. (2012). Anemia and erythrocytosis in patients after kidney transplantation. Transplant International, 25(10), 1013-1023. https://doi.org/10.1111/j.1432-2277.2012.01513.x
- Razeghi, E., Kaboli, A., Pezeshki, M. L., Meysamie, A. P., Khatami, M. R., & Khashayar, P. (2008). Risk factors of erythrocytosis post renal transplantation. Saudi Journal of Kidney Diseases and Transplantation, 19(4), 559-563. https://journals.lww.com/sjkd/Fulltext/2008/19040/Risk_Factors_of_Erythrocytosis_Post_Renal.7.aspx
- Vlahakos, D. V., Marathias, K. P., Agroyannis, B., & Madias, N. E. (2003). Posttransplant erythrocytosis. Kidney international, 63(4), 1187-1194. https://doi.org/10.1046/j.1523-1755.2003.00850.x